Natural Wollastonite-Derived Two-Dimensional Nanosheet Ni3Si2O5(OH)4 as a Novel Carrier of CdS for Efficient Photocatalytic H2 Generation
Abstract
:1. Introduction
2. Results and Discussion
2.1. Structure and Morphology of Ni3Si2O5(OH)4
2.2. Characterizations of CdS/NS
2.3. Binding Property of CdS/NS
2.4. Photocatalytic H2 Production Performance of CdS/NS
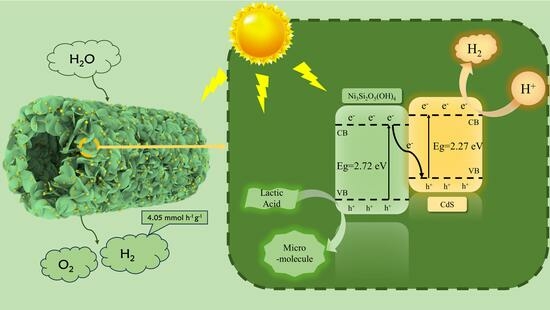
2.5. Photocatalytic Mechanism
3. Materials and Methods
3.1. Raw Materials and Reagents
3.2. Preparation of Ni3Si2O5(OH)4 Using Wollastonite as the Template
3.3. Preparation of CdS/Ni3Si2O5(OH)4
3.4. Photocatalytic H2 Evolution Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Xu, J.C.; Zhong, W.; Gao, D.D.; Wang, X.F.; Wang, P.; Yu, H.G. Phosphorus-enriched platinum diphosphide nanodots as a highly efficient cocatalyst for photocatalytic H2 evolution of CdS. Chem. Eng. J. 2022, 439, 135758. [Google Scholar] [CrossRef]
- Dong, Y.J.; Hu, Q.Y.; Li, B.N.; Li, X.H.; Chen, M.X.; Zhang, M.Y.; Feng, Y.; Ding, Y. Aminated silicon dioxide enriching iron-containing polyoxometalate catalyst confined in CdS for efficient H2 evolution. Appl. Catal. B-Environ. 2022, 304, 120998. [Google Scholar] [CrossRef]
- Li, C.Q.; Du, X.; Jiang, S.; Liu, Y.Z.; Niu, L.; Liu, Z.Y.; Yi, S.S.; Yue, X.Z. Constructing Direct Z-Scheme Heterostructure by Enwrapping ZnIn2S4 on CdS Hollow Cube for Efficient Photocatalytic H2 Generation. Adv. Sci. 2022, 9, e2201773. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.G.; Ng, K.H.; Zhu, Y.C.; Zhang, Y.Z.; Li, Z.X.; Xu, S.; Huang, J.Y.; Hu, J.; Chen, Z.; Cai, W.L.; et al. Mo-activated VC as effective cocatalyst for an enhanced photocatalytic hydrogen evolution activity of CdS. Chem. Eng. J. 2023, 452, 139325. [Google Scholar] [CrossRef]
- Wang, Y.R.; Zhao, J.J.; Hou, W.Q.; Xu, Y.M. Decoration of CdS nanowires with Ni3S4 nanoballs enhancing H2 and H2O2 production under visible light. Appl. Catal. B Environ. 2022, 310, 121350. [Google Scholar] [CrossRef]
- Tao, J.N.; Wang, M.Y.; Zhang, X.Z.; Lu, L.; Tang, H.; Liu, Q.Q.; Lei, S.Y.; Qiao, G.J.; Liu, G.W. A novel CoP@AAH cocatalyst leads to excellent stability and enhanced photocatalytic H2 evolution of CdS by structurally separating the photogenerated carriers. Appl. Catal. B Environ. 2023, 320, 122004. [Google Scholar] [CrossRef]
- Zhang, D.D.; Teng, J.; Yang, H.L.; Fang, Z.; Song, K.; Wang, L.; Hou, H.L.; Lu, X.L.; Bowen, C.R.; Yang, W.Y. Air-condition process for scalable fabrication of CdS/ZnS 1D/2D heterojunctions toward efficient and stable photocatalytic hydrogen production. Carbon Energy 2022, 5, e277. [Google Scholar] [CrossRef]
- Hao, P.L.; Cao, Y.L.; Ning, X.E.; Chen, R.Q.; Xie, J.; Hu, J.D.; Lu, Z.J.; Hao, A.Z. Rational design of CdS/BiOCl S-scheme heterojunction for effective boosting piezocatalytic H2 evolution and pollutants degradation performances. J. Colloid Interface Sci. 2023, 639, 343–354. [Google Scholar] [CrossRef]
- Rao, V.N.; Kwon, H.; Lee, Y.; Ravi, P.; Ahn, C.W.; Kim, K.; Yang, J.M. Synergistic integration of MXene nanosheets with CdS@TiO2 core@shell S-scheme photocatalyst for augmented hydrogen generation. Chem. Eng. J. 2023, 471, 144490. [Google Scholar]
- Liu, H.; Cheng, D.G.; Chen, F.Q.; Zhan, X.L. 2D Porous N-Deficient g-C3N4 Nanosheet Decorated with CdS Nanoparticles for Enhanced Visible-Light-Driven Photocatalysis. ACS Sustain. Chem. Eng. 2020, 8, 16897–16904. [Google Scholar] [CrossRef]
- Wang, W.; Fang, J.J. Mesoporous SiO2-derived g-C3N4@CdS core-shell heteronanostructure for efficient and stable photocatalytic H2 production. Ceram. Int. 2020, 46, 2384–2391. [Google Scholar] [CrossRef]
- Zhou, X.X.; Chen, H.R.; Sun, Y.Y.; Zhang, K.; Fan, X.Q.; Zhu, Y.; Chen, Y.; Tao, G.J.; Shi, J.L. highly efficient light-induced hydrogen evolution from a stable Pt/CdS NPs-co-loaded hierarchically porous zeolite beta. Appl. Catal. B Environ. 2014, 152–153, 271–279. [Google Scholar] [CrossRef]
- Li, C.Q.; Dong, X.G.; Zhu, N.Y.; Zhang, X.G.; Yang, S.S.; Sun, Z.M.; Liu, Y.Y.; Zheng, S.L.; Dionysiou, D.D. Rational design of efficient visible-light driven photocatalyst through 0D/2D structural assembly: Natural kaolinite supported monodispersed TiO2 with carbon regulation. Chem. Eng. J. 2020, 396, 125311. [Google Scholar] [CrossRef]
- Li, H.F.; Zhang, J.H.; Zhang, Y.P.; Huang, H.W.; Ou, H.L.; Zhang, Y.H. In-situ adsorption-conversion recovery of heavy metal cadmium by natural clay mineral for multi-functional photocatalysis. Sep. Purif. Technol. 2023, 319, 124058. [Google Scholar] [CrossRef]
- Mehrabanpour, N.; Nezamzadeh-Ejhieh, A.; Ghattavi, S. The boosted photocatalytic effects of a zeolite supported CdS towards an antibiotic model pollutant: A brief kinetics study. Environ. Sci. Pollut. Res. Int. 2023, 30, 5089–5102. [Google Scholar] [CrossRef] [PubMed]
- Li, X.Y.; Fu, L.J.; Ouyang, J.; Yang, H.M. Microwave-assisted synthesis and interfacial features of CdS/kaolinite nanocomposite. Colloid. Surf. A 2014, 443, 72–79. [Google Scholar] [CrossRef]
- Nascimento, C.C.; Andrade, G.R.S.; Santos, O.S.; Neto, E.T.; Costa, S.S.L.; Gimenez, I.F. Biosilica from diatomaceous earth as support to CdS-mediated photocatalysis in dry and aqueous phase. Mater. Des. 2017, 127, 8–14. [Google Scholar] [CrossRef]
- Zhang, Y.; Fu, L.J.; Shu, Z.; Yang, H.M.; Tang, A.D.; Jiang, T. Substitutional Doping for Aluminosilicate Mineral and Superior Water Splitting Performance. Nanoscale Res. Lett. 2017, 12, 456. [Google Scholar] [CrossRef]
- Peng, K.; Zuo, L.J.; Wang, Y.H.; Ye, J.Y.; Wang, H.J.; Jia, Y.L.; Niu, M.; Su, L.; Zhuang, L.; Li, X.Y. Boosting photocatalytic hydrogen evolution over CdS/MoS2 on the graphene/montmorillonite composites. Appl. Clay Sci. 2023, 236, 106855. [Google Scholar] [CrossRef]
- Wang, L.; Chen, K.Y.; Gao, Z.Q.; Wang, Q.Z. Synthesis of Ni-silicate superficially modified CdS and its highly improved photocatalytic hydrogen production. Appl. Surf. Sci. 2020, 529, 147217. [Google Scholar] [CrossRef]
- Lin, S.; Li, S.T.; Zhang, Y.H.; Ma, T.Y.; Huang, H.W. All-in-one polarized Cd/CdS/halloysite ferroelectric hybrid for exceptional photocatalytic hydrogen evolution. J. Mater. Chem. A 2020, 44, 88–97. [Google Scholar] [CrossRef]
- Chen, P.; Zeng, S.L.; Zhao, Y.L.; Kang, S.C.; Zhang, T.T.; Song, S.X. Synthesis of unique-morphological hollow microspheres of MoS2@montmorillonite nanosheets for the enhancement of photocatalytic activity and cycle stability. J. Mater. Sci. Technol. 2020, 41, 88–97. [Google Scholar] [CrossRef]
- Yu, Y.S.; Yang, M.N.; Yan, Z.L.; Li, T.T.; Jing, Q.S.; Liu, P.; Xu, B.; Cao, J.L. Regulation of hierarchically porous structures based on multi-scale nanosheets derived from kaolinite for enhanced adsorption. Appl. Clay Sci. 2021, 200, 105895. [Google Scholar] [CrossRef]
- Zhan, G.W.; Zeng, H.C. Topological Transformations of Core-Shell Precursors to Hierarchically Hollow Assemblages of Copper Silicate Nanotubes. ACS Appl. Mater. Interfaces 2017, 9, 37210–37218. [Google Scholar] [CrossRef] [PubMed]
- Vaughn, A.E.; Montenegro, A.; Howard, E.S.; Mammetkuliyev, M.; Falcon, S.; Mecklenburg, M.; Melot, B.C.; Benderskii, A.V. Vibrational Sum Frequency Generation Spectroscopy of Surface Hydroxyls on Nickel Phyllosilicate Nanoscrolls. J. Phys. Chem. Lett. 2021, 12, 10366–10371. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Jin, R.X.; Song, S.Y.; Xing, Y. Synthesis of flower-like nickel oxide/nickel silicate nanocomposites and their enhanced electrochemical performance as anode materials for lithium batteries. Mater. Lett. 2013, 93, 5–8. [Google Scholar] [CrossRef]
- Yang, Y.; Liang, Q.Q.; Li, J.H.; Zhuang, Y.; He, Y.H.; Bai, B.; Wang, X. Ni3Si2O5(OH)4 multi-walled nanotubes with tunable magnetic properties and their application as anode materials for lithium batteries. Nano Res. 2011, 4, 882–890. [Google Scholar] [CrossRef]
- Wang, Y.B.; Lin, F.; Shang, B.; Peng, B.; Deng, Z.W. Self-template synthesis of nickel silicate and nickel silicate/nickel composite nanotubes and their applications in wastewater treatment. J. Colloid Interface Sci. 2018, 522, 191–199. [Google Scholar] [CrossRef]
- Liu, J.Y.; Chen, T.T.; Jian, P.M.; Wang, L.X. Hierarchical hollow nickel silicate microflowers for selective oxidation of styrene. J. Colloid Interface Sci. 2019, 553, 606–612. [Google Scholar] [CrossRef]
- Wang, S.B.; Guan, B.Y.; Lu, Y.; Wen, X.; Luo, D. Formation of Hierarchical In2S3-CdIn2S4 Heterostructured Nanotubes for Efficient and Stable Visible Light CO2 Reduction. J. Am. Chem. Soc. 2017, 139, 17305–17308. [Google Scholar] [CrossRef]
- Xu, Z.X.; Zhang, G.F.; Lu, C.B.; Tian, H.; Xi, X.; Liu, R.L.; Wu, D.Q. Molybdenum carbide nanoparticle decorated hierarchical tubular carbon superstructures with vertical nanosheet arrays for efficient hydrogen evolution. J. Mater. Chem. A 2018, 6, 18833–18838. [Google Scholar] [CrossRef]
- Zhou, R.; Wang, J.; Wang, X.; Zhang, H.; Sun, S.J.; Li, Y.Z.; Ding, H.; Chen, D.M.; Li, W.; Zhang, J.M.; et al. Superhydrophilic wollastonite-nanoTiO2 composite photocatalyst prepared by a wet grinding method: The effects of carriers and their application in the self-cleaning coatings. Ceram. Int. 2022, 48, 13770–13779. [Google Scholar] [CrossRef]
- Chen, L.N.; Wang, X.N.; Chen, Y.W.; Zhuang, Z.Y.; Chen, F.F.; Zhu, Y.J.; Yu, Y. Recycling heavy metals from wastewater for photocatalytic CO2 reduction. Chem. Eng. J. 2020, 402, 125922. [Google Scholar] [CrossRef]
- Zhang, G.J.; Liu, J.W.; Xu, Y.; Sun, Y.H. Ordered mesoporous Ni/Silica-carbon as an efficient and stable catalyst for CO2 reforming of methane. Int. J. Hydrogen Energy 2019, 44, 4809–4820. [Google Scholar] [CrossRef]
- Jia, Z.G.; Han, C.; Wu, L.Y.; Zhang, D.Q.; Li, M. Biotemplated synthesis of hollow nickel silicate fiber for organic dye contaminants and its selective adsorption. Colloid. Surf. A 2022, 648, 129219. [Google Scholar] [CrossRef]
- Xiao, C.M.; Lin, J.M. PAMPS-graft-Ni3Si2O5OH4 multiwalled nanotubes as a novel nano-sorbent for the effective removal of Pb(ii) ions. RSC Adv. 2020, 10, 7619–7627. [Google Scholar] [CrossRef]
- Karikalan, N.; Velmurugan, M.; Chen, S.M.; Karuppiah, C. Modern Approach to the Synthesis of Ni(OH)2 Decorated Sulfur Doped Carbon Nanoparticles for the Nonenzymatic Glucose Sensor. ACS Appl. Mater. Interfaces 2016, 8, 22545–22553. [Google Scholar] [CrossRef]
- Zhao, M.Y.; Qin, J.Y.; Wang, N.; Zhang, Y.F.; Cui, H. Reconstruction of surface oxygen vacancy for boosting CO2 photoreduction mediated by BiOBr/CdS heterojunction. Sep. Purif. Technol. 2024, 329, 125179. [Google Scholar] [CrossRef]
- Song, S.; Yao, S.K.; Cao, J.H.; Di, L.; Wu, G.J.; Guan, N.J.; Li, L.D. Heterostructured Ni/NiO composite as a robust catalyst for the hydrogenation of levulinic acid to γ-valerolactone. Appl. Catal. B Environ. 2017, 217, 115–124. [Google Scholar] [CrossRef]
- Zhang, F.; Ji, R.J.; Liu, Y.H.; Li, Z.J.; Liu, Z.; Lu, S.C.; Wang, Y.T.; Wu, X.L.; Jin, H.; Cai, B.P. Defect-rich engineering and F dopant Co-modulated NiO hollow dendritic skeleton as a self-supported electrode for high-current density hydrogen evolution reaction. Chem. Eng. J. 2020, 401, 126037. [Google Scholar] [CrossRef]
- Pal, N.; Banerjee, S.; Choi, E.; Cho, E.B. Facile One-Pot Synthesis of Yolk-Shell Structured Ni Doped Mesoporous Silica and Its Application in Enzyme-Free Glucose Sensor. ChemistrySelect 2018, 3, 6029–6034. [Google Scholar] [CrossRef]
- Yuan, W.Q.; Kuang, J.Z.; Yu, M.M.; Huang, Z.Y.; Zou, Z.L.; Zhu, L.P. Facile preparation of MoS2@Kaolin composite by one-step hydrothermal method for efficient removal of Pb(II). J. Hazard. Mater. 2021, 405, 124261. [Google Scholar] [CrossRef] [PubMed]
- Abu-Zied, B.M.; Asiri, A.M. The role of alkali promoters in enhancing the direct N2O decomposition reactivity over NiO catalysts. Chin. J. Catal. 2015, 36, 1837–1845. [Google Scholar] [CrossRef]
- Wang, P.; Li, C.; Dong, S.; Ge, X.; Zhang, P.; Miao, X.; Wang, R.; Zhang, Z.; Yin, L. Hierarchical NiCo2S4@NiO Core–Shell Heterostructures as Catalytic Cathode for Long-Life Li-O2 Batteries. Adv. Energy. Mater. 2019, 9, 1900788. [Google Scholar] [CrossRef]
- Hu, Y.; Hao, X.Q.; Cui, Z.W.; Zhou, J.; Chu, S.Q.; Wang, Y.; Zou, Z.G. Enhanced photocarrier separation in conjugated polymer engineered CdS for direct Z-scheme photocatalytic hydrogen evolution. Appl. Catal. B Environ. 2020, 260, 118131. [Google Scholar] [CrossRef]
- Zhou, M.; Wang, S.B.; Yang, P.J.; Huang, C.J.; Wang, X.C. Boron Carbon Nitride Semiconductors Decorated with CdS Nanoparticles for Photocatalytic Reduction of CO2. ACS Catal. 2018, 8, 4928–4936. [Google Scholar] [CrossRef]
- Mohanraj, V.; Jayaprakash, R.; Chandrasekaran, J.; Robert, R.; Sangaiya, P. Influence of pH on particle size, band-gap and activation energy of CdS nanoparticles synthesized at constant frequency ultrasonic wave irradiation. Mater. Sci. Semicond Process 2017, 66, 131–139. [Google Scholar] [CrossRef]
- Ren, X.X.; Zhao, G.L.; Li, H.; Wu, W.; Han, G.R. The effect of different pH modifier on formation of CdS nanoparticles. J. Alloys Compd. 2008, 465, 534–539. [Google Scholar] [CrossRef]
- Güy, N. Directional transfer of photocarriers on CdS/g-C3N4 heterojunction modified with Pd as a cocatalyst for synergistically enhanced photocatalytic hydrogen production. Appl. Surf. Sci. 2020, 522, 146442. [Google Scholar] [CrossRef]
- Lang, D.; Cheng, F.Y.; Xiang, Q.J. Enhancement of photocatalytic H2-production activity of CdS nanorods by cobalt-based cocatalysts modification. Catal. Sci. Technol. 2016, 6, 6207–6216. [Google Scholar] [CrossRef]
- Wang, Y.; Peng, J.R.; Xu, Y.F.; Zhao, R.Y.; Han, J.S.; Wang, L. Facile fabrication of CdSe/CuInS2 microflowers with efficient photocatalytic hydrogen production activity. Int. J. Hydrogen Energy 2022, 47, 8294–8302. [Google Scholar] [CrossRef]
- Lv, R.G.; Ye, K.; Zhang, W.Y.; Chen, H.Y.; Zhao, R.F.; Wu, H.Y.; Chen, M. Homologous heterostructure CdSe/CdS nanoflowers to enhance photocatalytic hydrogen production. Colloids Surf. A. 2024, 684, 133143. [Google Scholar] [CrossRef]
- Guo, J.L.; Liang, Y.H.; Liu, L.; Hu, J.S.; Wang, H.; An, W.J.; Cui, W.Q. Noble-metal-free CdS/Ni-MOF composites with highly efficient charge separation for photocatalytic H2 evolution. Appl. Surf. Sci. 2020, 522, 146356. [Google Scholar] [CrossRef]
- Belakehal, R.; Güy, N.; Atacan, K.; Megriche, A.; Özacar, M. Emerging n-p-n Mn0.2Cd0.8S/CoFe2O4/rGO S-scheme heterojunction for synergistically improved photocatalytic H2 production. Mater. Chem. Phy. 2023, 310, 128453. [Google Scholar] [CrossRef]
- Bellamkonda, S.; Chakma, C.; Guru, S.; Neppolian, B.; Rao, G.R. Rational design of plasmonic Ag@CoFe2O4/g-C3N4 p-n heterojunction photocatalysts for efficient overall water splitting. Int. J. Hydrogen Energy 2022, 47, 18708–18724. [Google Scholar] [CrossRef]
- Chang, Y.C.; Chiao, Y.C.; Chang, C.J. Synthesis of g-C3N4@ZnIn2S4 Heterostructures with Extremely High Photocatalytic Hydrogen Production and Reusability. Catalysts 2023, 13, 118. [Google Scholar] [CrossRef]
- Shi, X.; Xu, J.P.; Shi, S.B.; Zhang, X.S.; Li, S.B.; Wang, C.; Wang, X.L.; Li, L.L.; Li, L. Effect of CdS modification on photoelectric properties of TiO2/PbS quantum dots bulk heterojunction. J. Phys. Chem. Solids 2016, 93, 33–39. [Google Scholar] [CrossRef]
- Yang, F.L.; Zhang, Q.; Zhang, J.H.; Zhang, L.; Cao, M.T.; Dai, W.L. Embedding Pt nanoparticles at the interface of CdS/NaNbO3 nanorods heterojunction with bridge design for superior Z-Scheme photocatalytic hydrogen evolution. Appl. Catal. B Environ. 2020, 278, 119290. [Google Scholar] [CrossRef]
- Zhang, W.J.; Zhou, X.Z.; Huang, J.Z.; Zhang, S.W.; Xu, X.J. Noble metal-free core-shell CdS/iron phthalocyanine Z-scheme photocatalyst for enhancing photocatalytic hydrogen evolution. J. Mater. Sci. Technol. 2022, 115, 199–207. [Google Scholar] [CrossRef]
- Wang, G.H.; Dou, K.; Cao, H.P.; Du, R.X.; Liu, J.L.; Tsidaeva, N.; Wang, W. Designing Z-scheme CdS/WS2 heterojunctions with enhanced photocatalytic degradation of organic dyes and photoreduction of Cr (VI): Experiments, DFT calculations and mechanism. Sep. Purif. Technol. 2022, 291, 120976. [Google Scholar] [CrossRef]
- Li, Y.Y.; Wei, Z.H.; Fan, J.B.; Li, Z.J.; Yao, H.C. Photocatalytic CO2 reduction activity of Z-scheme CdS/CdWO4 catalysts constructed by surface charge directed selective deposition of CdS. Appl. Surf. Sci. 2019, 483, 442–452. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, Y.N.; Zhang, B.H.; Deng, D.; Ning, J.G.; Liu, H.; Xue, S.Q.; Zhang, F.C.; Liu, X.H.; Zhang, W.H. Electronic structure and optical properties of CdS/BiOI heterojunction improved by oxygen vacancies. J. Alloys Compd. 2023, 955, 170235. [Google Scholar] [CrossRef]









| Catalyst | Light Source | Mcatalyst | Vreaction solution | Hydrogen Production Rate | Ref. |
|---|---|---|---|---|---|
| Pd–CdS/g-C3N4 | 300 W Xe (λ > 420 nm) | 50 mg | 100 mL | 293.0 μmol·g−1 h−1 | [49] |
| CdS-Co3O4 | 350 W Xe (λ > 420 nm) | 0.05 g | 80 mL | 150.7 μmol h−1 | [50] |
| CdSe/CuInS2 | 300 W Xe | 10 mg | 100 mL | 10610.37 μmol·g−1 h−1 | [51] |
| CdSe/CdS | 300W Xe (λ > 420 nm) | 10 mg | 100 mL | 16.03 mmol·g−1 h−1 | [52] |
| CdS/Ni-MOF | 300W Xe (λ > 420 nm) | 30 mg | 60 mL | 7.83 mmol·g−1 h−1 | [53] |
| Mn0.2Cd0.8S/CoFe2O4/rGO | 300W Xe (λ > 420 nm) | 50 mg | 100 mL | 133.5 μmol·g−1 h−1 | [54] |
| Ag@CoFe2O4/g-C3N4 | 300W Xe (λ > 420 nm) | 5 mg | 50 mL | 335 μmol·g−1 h−1 | [55] |
| g-C3N4@ZnIn2S4 | 5 W blue LED (λmax = 420 nm) | 50 mg | 50 mL | 2377.6 μmol·g−1 h−1 | [56] |
| R-TiO2/n-TiO2 | 300W Xe (λ > 420 nm) | 30 mg | 100 mL | 4.05 mmol h−1 g−1 | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, J.; Zhou, R.; Tu, Y.; Ma, R.; Chen, D.; Ding, H. Natural Wollastonite-Derived Two-Dimensional Nanosheet Ni3Si2O5(OH)4 as a Novel Carrier of CdS for Efficient Photocatalytic H2 Generation. Catalysts 2024, 14, 183. https://doi.org/10.3390/catal14030183
Ma J, Zhou R, Tu Y, Ma R, Chen D, Ding H. Natural Wollastonite-Derived Two-Dimensional Nanosheet Ni3Si2O5(OH)4 as a Novel Carrier of CdS for Efficient Photocatalytic H2 Generation. Catalysts. 2024; 14(3):183. https://doi.org/10.3390/catal14030183
Chicago/Turabian StyleMa, Jiarong, Run Zhou, Yu Tu, Ruixin Ma, Daimei Chen, and Hao Ding. 2024. "Natural Wollastonite-Derived Two-Dimensional Nanosheet Ni3Si2O5(OH)4 as a Novel Carrier of CdS for Efficient Photocatalytic H2 Generation" Catalysts 14, no. 3: 183. https://doi.org/10.3390/catal14030183