Hydrochloric Acid Catalyzed Hydrothermal Treatment to Recover Phosphorus from Municipal Sludge
Abstract
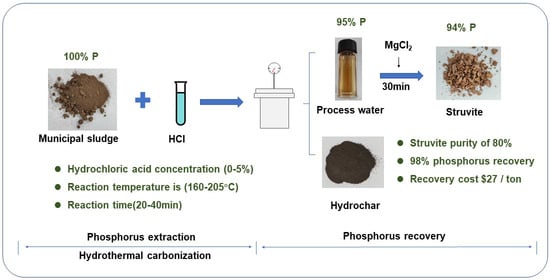
:1. Introduction
2. Results and Discussion
2.1. Extraction and Species of Phosphorus
2.1.1. Effect of Acid Addition
2.1.2. Effect of Temperature and Hold Time
2.2. Recovery of Phosphorus
2.3. Evaluation of Hydrochar
2.4. Economic Outlook of the Process
3. Materials and Methods
3.1. Municipal Sludge
3.2. Hydrothermal Carbonization of Sludge
3.3. Characterization of Product
3.4. P Recovery via Struvite Precipitation
3.5. Economic Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Meng, X.; Huang, Q.; Xu, J.; Gao, H.; Yan, J. A review of phosphorus recovery from different thermal treatment products of sewage sludge. Waste Dispos. Sustain. Energy 2019, 1, 99–115. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, C.; Gray, E.M.; Boyd, S.E.; Yang, H.; Zhang, D. Roles of biochar in improving phosphorus availability in soils: A phosphate adsorbent and a source of available phosphorus. Geoderma 2016, 276, 1–6. [Google Scholar] [CrossRef]
- Wang, H.; Yang, Z.; Li, X.; Liu, Y. Distribution and transformation behaviors of heavy metals and phosphorus during hydrothermal carbonization of sewage sludge. Environ. Sci. Pollut. Res. Int. 2020, 27, 17109–17122. [Google Scholar] [CrossRef] [PubMed]
- van Dijk, K.C.; Lesschen, J.P.; Oenema, O. Phosphorus flows and balances of the European Union Member States. Sci. Total Environ. 2016, 542, 1078–1093. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Ye, Y.; Jiang, Z.; Ying, Z.; Ji, S.; Chen, W.; Wang, B.; Dou, B. Enhanced transformation of phosphorus (P) in sewage sludge to hydroxyapatite via hydrothermal carbonization and calcium-based additive. Sci. Total Environ. 2020, 738, 139786. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Jiang, Z.; Ying, Z.; Ye, Y.; Chen, W.; Wang, B.; Dou, B. Migration and Transformation of Phosphorus during Hydrothermal Carbonization of Sewage Sludge: Focusing on the Role of pH and Calcium Additive and the Transformation Mechanism. ACS Sustain. Chem. Eng. 2020, 8, 7806–7814. [Google Scholar] [CrossRef]
- Parshetti, G.K.; Liu, Z.; Jain, A.; Srinivasan, M.P.; Balasubramanian, R. Hydrothermal carbonization of sewage sludge for energy production with coal. Fuel 2013, 111, 201–210. [Google Scholar] [CrossRef]
- Czerwińska, K.; Śliz, M.; Wilk, M. Hydrothermal carbonization process: Fundamentals, main parameter characteristics and possible applications including an effective method of SARS-CoV-2 mitigation in sewage sludge. A review. Renew. Sustain. Energy Rev. 2022, 154, 111873. [Google Scholar] [CrossRef]
- Numviyimana, C.; Warchoł, J.; Khalaf, N.; Leahy, J.J.; Chojnacka, K. Phosphorus recovery as struvite from hydrothermal carbonization liquor of chemically produced dairy sludge by extraction and precipitation. J. Environ. Chem. Eng. 2022, 10, 106947. [Google Scholar] [CrossRef]
- Liu, Y.; Li, B.; Guo, D.; Munir, M.T.; Song, L.; Wu, X.; Huang, Y. Feasibility of using different hydrothermal processes for sewage sludge management in China. Sci. Total Environ. 2022, 838, 156154. [Google Scholar] [CrossRef]
- Hudcová, H.; Vymazal, J.; Rozkošný, M. Present restrictions of sewage sludge application in agriculture within the European Union. Soil Water Res. 2019, 14, 104–120. [Google Scholar] [CrossRef]
- Wang, T.; Zhai, Y.; Zhu, Y.; Li, C.; Zeng, G. A review of the hydrothermal carbonization of biomass waste for hydrochar formation: Process conditions, fundamentals, and physicochemical properties. Renew. Sustain. Energy Rev. 2018, 90, 223–247. [Google Scholar] [CrossRef]
- Funke, A.; Ziegler, F. Hydrothermal carbonization of biomass: A summary and discussion of chemical mechanisms for process engineering. Biofuels Bioprod. Biorefin. 2010, 4, 160–177. [Google Scholar] [CrossRef]
- Moller, M.; Nilges, P.; Harnisch, F.; Schroder, U. Subcritical water as reaction environment: Fundamentals of hydrothermal biomass transformation. ChemSusChem 2011, 4, 566–579. [Google Scholar] [CrossRef] [PubMed]
- Sarrion, A.; Diaz, E.; de la Rubia, M.A.; Mohedano, A.F. Fate of nutrients during hydrothermal treatment of food waste. Bioresour. Technol. 2021, 342, 125954. [Google Scholar] [CrossRef]
- Xia, Y.; Luo, H.; Li, D.; Chen, Z.; Yang, S.; Liu, Z.; Yang, T.; Gai, C. Efficient immobilization of toxic heavy metals in multi-contaminated agricultural soils by amino-functionalized hydrochar: Performance, plant responses and immobilization mechanisms. Environ. Pollut. 2020, 261, 114217. [Google Scholar] [CrossRef] [PubMed]
- Waldmüller, W.; Herdzik, S.; Gaderer, M. Combined filtration and oxalic acid leaching for recovering phosphorus from hydrothermally carbonized sewage sludge. J. Environ. Chem. Eng. 2021, 9, 104800. [Google Scholar] [CrossRef]
- Wang, T.; Zhai, Y.; Zhu, Y.; Peng, C.; Wang, T.; Xu, B.; Li, C.; Zeng, G. Feedwater pH affects phosphorus transformation during hydrothermal carbonization of sewage sludge. Bioresour. Technol. 2017, 245, 182–187. [Google Scholar] [CrossRef]
- Ekpo, U.; Ross, A.B.; Camargo-Valero, M.A.; Fletcher, L.A. Influence of pH on hydrothermal treatment of swine manure: Impact on extraction of nitrogen and phosphorus in process water. Bioresour. Technol. 2016, 214, 637–644. [Google Scholar] [CrossRef]
- Qaramaleki, V.S.; Villamil, J.A.; Mohedano, A.F.; Coronella, C.J. Factors Affecting Solubilization of Phosphorus and Nitrogen through Hydrothermal Carbonization of Animal Manure. ACS Sustain. Chem. Eng. 2020, 8, 12462–12470. [Google Scholar] [CrossRef]
- Han, X.; Wang, F.; Zhou, B.; Chen, H.; Yuan, R.; Liu, S.; Zhou, X.; Gao, L.; Lu, Y.; Zhang, R. Phosphorus complexation of sewage sludge during thermal hydrolysis with different reaction temperature and reaction time by P K-edge XANES and (31)P NMR. Sci. Total Environ. 2019, 688, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Lucian, M.; Merzari, F.; Gubert, M.; Messineo, A.; Volpe, M. Industrial-Scale Hydrothermal Carbonization of Agro-Industrial Digested Sludge: Filterability Enhancement and Phosphorus Recovery. Sustainability 2021, 13, 9343. [Google Scholar] [CrossRef]
- Shi, Y.; Chen, Z.; Cao, Y.; Fan, J.; Clark, J.H.; Luo, G.; Zhang, S. Migration and transformation mechanism of phosphorus in waste activated sludge during anaerobic fermentation and hydrothermal conversion. J. Hazard. Mater. 2021, 403, 123649. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhai, Y.; Li, S.; Liu, X.; Wang, B.; Liu, X.; Fan, Y.; Shi, H.; Li, C.; Zhu, Y. Thermal treatment of sewage sludge: A comparative review of the conversion principle, recovery methods and bioavailability-predicting of phosphorus. Chemosphere 2022, 291, 133053. [Google Scholar] [CrossRef] [PubMed]
- Becker, G.C.; Wust, D.; Kohler, H.; Lautenbach, A.; Kruse, A. Novel approach of phosphate-reclamation as struvite from sewage sludge by utilising hydrothermal carbonization. J. Environ. Manag. 2019, 238, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Shiba, N.C.; Ntuli, F. Extraction and precipitation of phosphorus from sewage sludge. Waste Manag. 2017, 60, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Daneshgar, S.; Callegari, A.; Capodaglio, A.; Vaccari, D. The Potential Phosphorus Crisis: Resource Conservation and Possible Escape Technologies: A Review. Resources 2018, 7, 37. [Google Scholar] [CrossRef]
- Heilmann, S.M.; Molde, J.S.; Timler, J.G.; Wood, B.M.; Mikula, A.L.; Vozhdayev, G.V.; Colosky, E.C.; Spokas, K.A.; Valentas, K.J. Phosphorus reclamation through hydrothermal carbonization of animal manures. Environ. Sci. Technol. 2014, 48, 10323–10329. [Google Scholar] [CrossRef]
- Dai, L.; Yang, B.; Li, H.; Tan, F.; Zhu, N.; Zhu, Q.; He, M.; Ran, Y.; Hu, G. A synergistic combination of nutrient reclamation from manure and resultant hydrochar upgradation by acid-supported hydrothermal carbonization. Bioresour. Technol. 2017, 243, 860–866. [Google Scholar] [CrossRef]
- Shi, Y.; Luo, G.; Rao, Y.; Chen, H.; Zhang, S. Hydrothermal conversion of dewatered sewage sludge: Focusing on the transformation mechanism and recovery of phosphorus. Chemosphere 2019, 228, 619–628. [Google Scholar] [CrossRef]
- Zheng, X.; Shen, M.; Ying, Z.; Feng, Y.; Wang, B.; Dou, B. Correlating phosphorus transformation with process water during hydrothermal carbonization of sewage sludge via experimental study and mathematical modelling. Sci. Total Environ. 2022, 807, 150750. [Google Scholar] [CrossRef]
- Huang, R.; Tang, Y. Evolution of phosphorus complexation and mineralogy during (hydro)thermal treatments of activated and anaerobically digested sludge: Insights from sequential extraction and P K-edge XANES. Water Res. 2016, 100, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Zhai, Y.; Zhu, Y.; Peng, C.; Xu, B.; Wang, T.; Li, C.; Zeng, G. Influence of temperature on nitrogen fate during hydrothermal carbonization of food waste. Bioresour. Technol. 2018, 247, 182–189. [Google Scholar] [CrossRef]
- Zhao, X.; Becker, G.C.; Faweya, N.; Rodriguez Correa, C.; Yang, S.; Xie, X.; Kruse, A. Fertilizer and activated carbon production by hydrothermal carbonization of digestate. Biomass Convers. Biorefin. 2017, 8, 423–436. [Google Scholar] [CrossRef]
- He, C.; Wang, K.; Yang, Y.; Amaniampong, P.N.; Wang, J.Y. Effective nitrogen removal and recovery from dewatered sewage sludge using a novel integrated system of accelerated hydrothermal deamination and air stripping. Environ. Sci. Technol. 2015, 49, 6872–6880. [Google Scholar] [CrossRef] [PubMed]
- Moulessehoul, A.; Gallart-Mateu, D.; Harrache, D.; Djaroud, S.; de la Guardia, M.; Kameche, M. Conductimetric study of struvite crystallization in water as a function of pH. J. Cryst. Growth 2017, 471, 42–52. [Google Scholar] [CrossRef]
- Szogi, A.A.; Vanotti, M.B.; Hunt, P.G. Phosphorus recovery from pig manure solids prior to land application. J. Environ. Manag. 2015, 157, 1–7. [Google Scholar] [CrossRef]
- Xiong, J.-B.; Pan, Z.-Q.; Xiao, X.-F.; Huang, H.-J.; Lai, F.-Y.; Wang, J.-X.; Chen, S.-W. Study on the hydrothermal carbonization of swine manure: The effect of process parameters on the yield/properties of hydrochar and process water. J. Anal. Appl. Pyrolysis 2019, 144, 104692. [Google Scholar] [CrossRef]
- Wang, J.-X.; Chen, S.-W.; Lai, F.-Y.; Liu, S.-Y.; Xiong, J.-Y.; Zhou, C.-F.; Yi, Y.; Huang, H.-J. Microwave-assisted hydrothermal carbonization of pig feces for the production of hydrochar. J. Supercrit. Fluids 2020, 162, 104858. [Google Scholar] [CrossRef]
- Zhang, T.; He, X.; Deng, Y.; Tsang DC, W.; Jiang, R.; Becker, G.C.; Kruse, A. Phosphorus recovered from digestate by hydrothermal processes with struvite crystallization and its potential as a fertilizer. Sci. Total Environ. 2020, 698, 134240. [Google Scholar] [CrossRef]
- Vardanyan, A.; Kafa, N.; Konstantinidis, V.; Shin, S.G.; Vyrides, I. Phosphorus dissolution from dewatered anaerobic sludge: Effect of pHs, microorganisms, and sequential extraction. Bioresour. Technol. 2018, 249, 464–472. [Google Scholar] [CrossRef]
- Zhou, Z.; Chen, L.; Wu, Q.; Zheng, T.; Yuan, H.; Peng, N.; He, M. The valorization of biogas slurry with a pilot dual stage reverse osmosis membrane process. Chem. Eng. Res. Des. 2019, 142, 133–142. [Google Scholar] [CrossRef]
- GB/T 212-2008; Proximate Analysis of Coal. General Administration of Quality Supervision, Inspection and Quarantine. Standardization Administration of PRC: Beijing, China, 2009.
- Volpe, M.; Picone, A.; Luz, F.C.; Mosonik, M.C.; Volpe, R.; Messineo, A. Potential pitfalls on the scalability of laboratory-based research for hydrothermal carbonization. Fuel 2022, 315, 123189. [Google Scholar] [CrossRef]




| Proximately Analysis(wt.%) | Ultimately Analysis(wt.%) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Samples | Ash | Volatile | Fixed Carbon | C | H | N | S | O a | HHV b (MJ kg−1) | Solid Yield c | H/C | O/C | (N + O)/C |
| Dewatered sludge | 55.48 | 41.17 | 3.34 | 13.38 | 3.08 | 1.12 | 3.61 | 23.33 | 5.06 | \ | 2.76 | 1.31 | 1.83 |
| Hydrochar (0%HCl-205 °C-40 min) | 57.31 | 36.12 | 6.57 | 11.79 | 2.06 | 0.44 | 3.72 | 24.69 | 3.15 | 53.75 | 2.09 | 1.57 | 2.13 |
| 1-205-40 | 63.47 | 27.87 | 8.66 | 12.58 | 2.41 | 0.42 | 4.22 | 16.9 | 4.56 | 45.99 | 2.30 | 1.01 | 1.83 |
| 2-205-40 | 63.03 | 24.63 | 12.34 | 14.01 | 2.58 | 0.41 | 4.46 | 15.51 | 5.44 | 36.74 | 2.21 | 0.83 | 1.51 |
| 3-205-40 | 64.72 | 20.8 | 14.48 | 15.37 | 2.45 | 0.39 | 3.73 | 13.34 | 5.88 | 29.88 | 1.91 | 0.65 | 1.19 |
| 4-205-40 | 65.31 | 20.11 | 14.58 | 16.55 | 2.31 | 0.38 | 2.99 | 12.46 | 6.13 | 27.63 | 1.67 | 0.56 | 1.03 |
| 5-205-40 | 20.01 | 66.13 | 13.86 | 17.01 | 2.05 | 0.37 | 1.90 | 12.54 | 5.85 | 23.54 | 1.45 | 0.55 | 1.01 |
| Optimized hydrochar (5-195-25) | 65.97 | 19.75 | 14.28 | 17.15 | 2.12 | 0.38 | 1.95 | 12.44 | 6.00 | 24.09 | 1.48 | 0.54 | 0.75 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, K.; Xue, Y.; Zhai, Y.; Zhou, L.; Kang, J. Hydrochloric Acid Catalyzed Hydrothermal Treatment to Recover Phosphorus from Municipal Sludge. Catalysts 2024, 14, 65. https://doi.org/10.3390/catal14010065
Liu K, Xue Y, Zhai Y, Zhou L, Kang J. Hydrochloric Acid Catalyzed Hydrothermal Treatment to Recover Phosphorus from Municipal Sludge. Catalysts. 2024; 14(1):65. https://doi.org/10.3390/catal14010065
Chicago/Turabian StyleLiu, Kai, Yang Xue, Yawei Zhai, Lisong Zhou, and Jian Kang. 2024. "Hydrochloric Acid Catalyzed Hydrothermal Treatment to Recover Phosphorus from Municipal Sludge" Catalysts 14, no. 1: 65. https://doi.org/10.3390/catal14010065