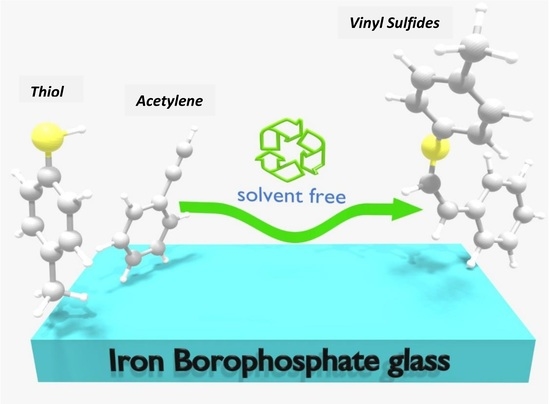
Iron-Borophosphate Glass-Catalyzed Regioselective Hydrothiolation of Alkynes under Green Conditions
Abstract
:1. Introduction
2. Results and Discussion
2.1. Iron-Borophosphate Glass Characterization
2.2. Regioselective Hydrothiolation of Alkynes
3. Materials and Methods
3.1. General Information
3.2. Glass Synthesis
3.3. General Procedure for the Preparation of Vinyl Sulfides
3.4. NMR Spectroscopic Data
- Styryl(p-tolyl)sulfane [25] (3a): Yield: 97%. (E/Z): 82/18. 1H NMR (200 MHz, CDCl3): δ = 7.54–7.06 (m, 9H); 6.85 (d, J = 15.5 Hz, 0.82 × 1H); 6.63 (d, J = 15.5 Hz, 0.82 × 1H); 6.54 (d, J = 10.8 Hz, 0.18 × 1H); 6.44 (d, J = 10.8 Hz, 0.18 × 1H); 2.34 (s, 0.82 × 3H); 2.30 (s, 0.18 × 3H) ppm. 13C NMR (75 MHz, CDCl3): δ = 137.5, 136.7, 132.8, 130.7, 130.6, 130.1, 130.0, 128.9, 128.8, 128.4, 127.5, 127.2, 127.1, 126.6, 126.1, 124.6, 21.2.
- Phenyl(styryl)sulfane [25] (3b): Yield: 82%. (E/Z): 67/33. 1H NMR (300 MHz, CDCl3): δ = 7.46 −7.14 (m, 10H); 6.80 (d, J = 15.5 Hz, 0.67 × 1H); 6.64 (d, J = 15.5 Hz, 0.67 × 1H); 6.50 (d, J = 10.8 Hz, 0.33 × 1H); 6.41 (d, J = 10.8 Hz, 0.33 × 1H) ppm. 13C NMR (75 MHz, CDCl3): δ = 136.6, 136.3, 135.3, 131.8, 130.1, 129.9, 129.2, 128.8, 128.7, 128.4, 127.6, 127.3, 127.2, 127.0, 126.1, 123.4 ppm.
- (E)-(4-chlorophenyl)(styryl)sulfane [47] (3c): Yield: 76%. (E/Z): 100/0. 1H NMR (300 MHz, CDCl3): δ = 7.33–7.24 (m, 9H); 6.82 (d, J = 15.4 Hz, 1H), 6.73 (d, J = 15.4 Hz, 1H). 13C NMR (75 MHz, CDCl3): δ = 136.3, 133.9, 133.0, 132.8, 131.0, 129.3, 128.7, 127.8, 126.1, 122.5 ppm.
- (4-bromophenyl)(styryl)sulfane [17] (3d): Yield: 65%. (E/Z): 70/30. 1H NMR (300 MHz, CDCl3): δ = 7.56–7.24 (m, 9H); 6.75 (d, J = 15.4 Hz, 0.70 × 1H); 6.82 (d, J = 15.4 × 2H); 6.63 (d, J = 10.6 Hz, 0.30 × 1H); 6.41 (d, J = 10.6 Hz, 0.30 × 1H) ppm. 13C NMR (75MHz, CDCl3): δ =136.3, 134.6, 133.0, 132.8, 132.2, 131.4, 131.1, 129.0, 128.7, 128.4, 128.2, 127.9, 127.4, 126.3, 126.1, 124.9, 122.2, 120.9 ppm.
- Naphthalen-2-yl(styryl)sulfane [48] (3e): Yield: 81%. (E/Z): 32/68. 1H NMR (300 MHz, CDCl3): δ = 7.87–7.11 (m, 12H); 6.87 (d, J = 15.3 Hz, 0.32 × 1H); 6.69 (d, J = 15.3 Hz, 0.32 × 1H); 6.55 (d, J = 10.5 Hz, 0.68 × 1H); 6.49 (d, J = 10.5 Hz, 0.68 × 1H) ppm. 13C NMR (75 MHz, CDCl3): δ = 136.5, 133.7, 133.6, 132.3, 132.2, 129.0, 128.9, 128.8, 128.5, 128.4, 128.2, 127.8, 127.7, 127.6, 127.5, 127.4, 127.3, 126.8, 126.6, 126.3, 126.1, 125.8, 125.7, 123.2 ppm.
- Cyclohexyl(styryl)sulfane [25] (3f): Yield: 73%. (E/Z): 10/90. 1H NMR (300 MHz, CDCl3) δ = 7.41 (d, J = 7.20 Hz, 2H); 7.30–7.10 (m, 3H); 6.69 (d, J = 15.6 Hz, 0.10 × 1H); 6.50 (d, J = 15.6 Hz, 0.10 × 1H); 6.36 (d, J = 10.8 Hz, 0.90 × 1H); 6.26 (d, J = 10.8 Hz, 0.90 × 1H); 2.87–2.77 (m, 1H); 2.02–1.31 (m, 10H) ppm. 13C NMR (75 MHz, CDCl3) δ = 137.1, 128.6, 128.2, 126.5, 125.9, 125.0, 47.8, 33.7, 29.7, 26.0, 25.6 ppm.
- Propyl(styryl)sulfane [30] (3g): Yield: 45%. (E/Z): 17/83. 1H NMR (300 MHz, CDCl3) δ = 7.48 (d, J = 6.20 Hz, 2H); 7.36–7.16 (m, 3H); 6.71 (d, J = 15.6 Hz, 0.17 × 1H); 6.50 (d, J = 15.6 Hz, 0.17 × 1H); 6.41 (d, J = 10.9 Hz, 0.83 × 1H); 6.23 (d, J = 10.9 Hz, 0.83 × 1H); 2.75 (t, J = 7.2 Hz, 2H); 1.77–1.65 (m, 2H); 1.02 (t, J = 7.2 Hz, 3H) ppm. 13C NMR (75 MHz, CDCl3) δ = 137.1, 128.6, 128.2, 127.7, 126.8, 126.6, 125.5, 125.4, 125.3, 37.9, 34.7, 23.6, 22.9, 13.4, 13.2 ppm.
- Butyl(styryl)sulfane [47] (3h): Yield: 40%. (E/Z): 12/88. 1H NMR (300 MHz, CDCl3) δ = 7.47 (d, J = 6.99 Hz, 2H); 7.36–7.16 (m, 3H); 6.71 (d, J = 15.6 Hz, 0.12 × 1H); 6.45 (d, J = 15.6 Hz, 0.12 × 1H); 6.41 (d, J = 10.9 Hz, 0.88 × 1H); 6.23 (d, J = 10.9 Hz, 0.88 × 1H); 2.77 (t, J = 7.5 Hz, 2H), 1.66 (quint, J = 7.5 Hz, 2H), 1.49–1,37 (m, 2H), 0.92 (t, J = 7.5 Hz, 3H) ppm. 13C NMR (75 MHz, CDCl3) δ = 137.1, 128.6, 128.2, 127.7, 126.5, 125.3, 35.6, 32.3, 21.7, 13.6 ppm.
- (Z)-3-(2-(p-tolylthio)vinyl)thiophene [49] (3i): Yield: 93%. (E/Z): 0/100. 1H NMR (300 MHz, CDCl3): δ = 7.49–7.47 (m, 1H); 7.37 (d, J = 8.2 Hz, 2H); 7.30–7.29 (m, 2H); 7.15 (d, J = 8.2 Hz, 2H); 6.56 (d, J = 10.5 Hz, 1H); 6.37 (d, J = 10.5 Hz, 1H); 2.34 (s, 3H) ppm. 13C NMR (75 MHz, CDCl3): δ = 137.9, 137.4, 132.3, 130.5, 130.0, 128.6, 125.7, 125.1, 123.7, 120.9, 21.1 ppm.
- (Z)-3-(2-((4-bromophenyl)thio)vinyl)thiophene (3j): Yield: 82%. (E/Z): 30/70. 1H NMR (300 MHz, CDCl3): δ = 7.48–7.43 (m, 3H); 7.32–7.7.26 (m, 4H); 6.80 (d, J = 15.3 Hz, 0.30 × 1H); 6.66 (d, J = 10.5 Hz, 0.70 × 1H); 6.64 (d, J = 15.3 Hz, 0.30 × 1H); 6.34 (d, J = 10.5 Hz, 0.70 × 1H) ppm. 13C NMR (75 MHz, CDCl3): δ = 138.8, 137.7, 135.2, 134.9, 132.4, 132.3, 131.5, 131.1, 128.6, 128.2, 126.6, 125.4, 124.8, 124.3, 123.5, 122.8, 127.4, 121.7, 121.3, 120.9 ppm.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brow, R.K. Nature of Alumina in Phosphate Glass: I, Properties of Sodium Aluminophosphate Glass. J. Am. Ceram. Soc. 1993, 76, 913–918. [Google Scholar] [CrossRef]
- Hoppe, U. A structural model for phosphate glasses. J. Non-Cryst. Solids 1996, 195, 138–147. [Google Scholar] [CrossRef]
- Tupberg, C.; Chandet, N.; Wattanavichan, K.; Randorn, C. Catalytic and antibacterial activities of novel colored zinc borophosphate glasses. RSC Adv. 2016, 6, 79602–79611. [Google Scholar] [CrossRef]
- Rafique, J.; Saba, S.; Frizon, T.E.A.; Braga, A.L. Fe3O4 Nanoparticles: A Robust and Magnetically Recoverable Catalyst for Direct C-H Bond Selenylation and Sulfenylation of Benzothiazoles. ChemistrySelect 2018, 3, 328–334. [Google Scholar] [CrossRef]
- Mojtahedi, M.M.; Abaee, M.S.; Rajabi, A.; Mahmoodi, P.; Bagherpoor, S. Recyclable superparamagnetic Fe3O4 nanoparticles for efficient catalysis of thiolysis of epoxides. J. Mol. Catal. A Chem. 2012, 361–362, 68–71. [Google Scholar] [CrossRef]
- Godoi, M.; Liz, D.G.; Ricardo, E.W.; Rocha, M.S.T.; Azeredo, J.B.; Braga, A.L. Magnetite (Fe3O4) nanoparticles: An efficient and recoverable catalyst for the synthesis of alkynyl chalcogenides (selenides and tellurides) from terminal acetylenes and diorganyl dichalcogenides. Tetrahedron 2014, 70, 3349–3354. [Google Scholar] [CrossRef]
- Kassaee, M.Z.; Motamedi, E.; Movassagh, B.; Poursadeghi, S. Iron-Catalyzed Formation of C–Se and C–Te Bonds through Cross Coupling of Aryl Halides with Se(0) and Te(0)/Nano-Fe3O4@GO. Synthesis 2013, 45, 2337–2342. [Google Scholar] [CrossRef]
- Palomba, M.; Bagnoli, L.; Marini, F.; Santi, C.; Sancineto, L. Recent advances in the chemistry of vinylchalcogenides. Phosphorus Sulfur Silicon Relat. Elem. 2016, 191, 235–244. [Google Scholar] [CrossRef]
- Beletskaya, I.P.; Ananikov, V.P. Transition-Metal-Catalyzed C-S, C-Se, and C-Te Bond Formation via Cross-Coupling and Atom-Economic Addition Reactions. Chem. Rev. 2011, 11, 1596–1636. [Google Scholar] [CrossRef]
- Pearson, W.H.; Lee, I.Y.; Mi, Y.; Stoy, P. Total Synthesis of the Kopsia Lapidilecta Alkaloid (±)-Lapidilectine B. J. Org. Chem. 2013, 69, 9109–9122. [Google Scholar] [CrossRef]
- Reddy, V.P.; Swapna, K.; Kumar, A.V.; Rao, K.R. Recyclable Nano Copper Oxide Catalyzed Stereoselective Synthesis of Vinyl Sulfides under Ligand-Free Conditions. Synlett 2009, 17, 2783–2788. [Google Scholar] [CrossRef]
- Kundu, D.; Chatterjee, T.; Ranu, B.C. Magnetically Separable CuFe2O4 Nanoparticles Catalyzed Ligand-Free C-S Coupling in Water: Access to (E)- and (Z)- Styrenyl- Heteroaryl and Sterically Hindered Aryl Sulfides. Adv. Synth. Cat. 2013, 355, 2285–2296. [Google Scholar] [CrossRef]
- Gonçalves, L.C.C.; Lima, D.B.; Borba, P.M.Y.; Perin, G.; Alves, D.; Jacob, R.G.; Lenardao, E.J. Glycerol/CuI/Zn as a recyclable catalytic system for synthesis of vinyl sulfides and tellurides. Tetrahedron Lett. 2013, 54, 3475–3480. [Google Scholar] [CrossRef] [Green Version]
- Rodygin, K.S.; Gyrdymova, Y.V.; Zarubaev, V.V. Synthesis of vinyl thioethers and bis-thioethenes from calcium carbide and disulfides. Mendeleev Commun. 2017, 27, 476–478. [Google Scholar] [CrossRef]
- Palacios, L.; Giuseppe, A.D.; Artigas, M.J.; Polo, V.; Lahoz, F.J.; Castarlenas, R.; Pérez-Torrente, J.; Oro, L.A. Mechanistic insight into the pyridine enhanced α-selectivity in alkyne hydrothiolation catalysed by quinolinolate-rhodium(ǀ)-N-heterocyclic carbine complexes. Catal. Sci. Technol. 2016, 6, 8548–8561. [Google Scholar] [CrossRef] [Green Version]
- Dondoni, A.; Marra, A. Metal-Catalyzed and Metal-Free Alkyne Hydrothiolation: Synthetic Aspects and Application Trends. Eur. J. Org. Chem. 2014, 2014, 3955–3969. [Google Scholar] [CrossRef]
- Chu, S.; Chung, J.; Park, J.E.; Chung, Y.K. Hydrothiolation of Alkenes and Alkynes Catalyzed by 3,4-Dimethyl-5-vinylthiazolium iodide and Poly (3,4-dimethyl-5-vinylthiazolium) iodide. ChemCatChem 2016, 8, 2476–2481. [Google Scholar]
- Silva, M.S.; Lara, R.G.; Marczewski, J.M.; Jacob, R.G.; Lenardão, E.J.; Perin, G. Synthesis of vinyl sulfides via hydrothiolation of alkynes using Al2O3/KF under solvent-free conditions. Tetrahedron Lett. 2008, 49, 1927–1930. [Google Scholar] [CrossRef]
- Rosa, C.H.; Peixoto, M.L.B.; Rosa, G.R.; Godoi, B.; Galetto, F.Z.; D’Oca, M.G.M.; Godoi, M. Sulfamic acid: An efficient and recyclable catalyst for the regioselective hydrothiolation of terminal alkenes and alkynes with thiols. Tetrahedron Lett. 2017, 58, 3777–3781. [Google Scholar] [CrossRef]
- Kondoh, A.; Takami, K.; Yorimitsu, H.; Oshima, K. Stereoselective Hydrothiolation of Alkynes Catalyzed by Cesium Base: Facile Access to (Z)-1-Alkenyl Sulfides. J. Org. Chem. 2005, 70, 6468–6473. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Chen, S.; Jiang, P.; Qi, H.; Deng, G.-J. Stereoselective Formation of Z- or E-Vinyl Thioethers from Arylthiols and Acetylenes under Transition-Metal-Free Condition. Eur. J. Org. Chem. 2013, 2013, 6878–6885. [Google Scholar] [CrossRef]
- Godoi, M.; Leitemberger, A.; Böhs, L.M.C.; Silveira, M.V.; Rafique, J.; D’Oca, M.G.M. Rice straw ash extract, an efficient solvent for regioselective hydrothiolation of alkynes. Environ. Chem. Lett. 2019, 17, 1441–1446. [Google Scholar] [CrossRef]
- Silveira, M.V.; Zandoná, G.; Leitemberger, V.; Böhs, L.M.C.; Lopes, T.J.; Martins, M.L.; Godoi, M. Water Extract of Rice Straw Ash: Experimental Design and Evaluation of Their Activity in the Hydrothiolation Reaction. Waste Biomass Valorization 2021, 12, 5041–5050. [Google Scholar] [CrossRef]
- Corma, A.; González-Arellano, C.; Iglesias, M.; Sánchez, F. Efficient synthesis of vinyl and alkyl sulfides via hydrothiolation of alkynes and electron-deficient olefins using soluble and heterogenized gold complexes catalysts. Appl. Catal. A Gen. 2010, 375, 49–54. [Google Scholar] [CrossRef]
- Riduan, S.N.; Ying, J.Y.; Zhang, Y. Carbon Dioxide Mediated Stereoselective Copper-Catalyzed Reductive Coupling of Alkynes and Thiols. Org. Lett. 2012, 14, 1780–1783. [Google Scholar] [CrossRef] [PubMed]
- Malyshev, D.A.; Scott, N.M.; Marion, N.; Stevens, E.D.; Ananikov, V.P.; Beletskaya, I.P.; Nolan, S.P. Homogeneous Nickel Catalysts for the Selective Transfer of a Single Arylthio Group in the Catalytic Hydrothiolation of Alkynes. Organometallics 2006, 25, 4462–4470. [Google Scholar] [CrossRef]
- Yang, Y.; Rioux, R.M. Highly stereoselective anti-Markovnikov hydrothiolation of alkynes and electron-deficient alkenes by a supported Cu-NHC complex. Green Chem. 2014, 16, 3916–3925. [Google Scholar] [CrossRef]
- Trostyanskaya, I.G.; Beletskaya, I.P. Regio- and Stereoselective Copper-Catalyzed Addition of Aromatic and Aliphatic Thiols to Terminal and Internal Nonactivated Alkynes. Synlett 2012, 23, 535–540. [Google Scholar]
- Rocha, M.S.T.; Rafique, J.; Saba, S.; Azeredo, J.B.; Back, D.; Godoi, M.; Braga, A.L. Regioselective Hydrothiolation of Terminal Acetylene catalyzed by Magnetite (Fe3O4) Nanoparticles. Synth. Comm. 2017, 47, 291–298. [Google Scholar] [CrossRef]
- Sarma, R.; Rajesh, N.; Prajapati, D. Indium(III) catalysed substrate selective hydrothiolation of terminal alkynes. Chem. Comm. 2012, 48, 4014–4016. [Google Scholar] [CrossRef]
- Zhao, H.; Peng, J.; Cai, M. Heterogeneous Hydrothiolation of Alkynes with Thiols Catalyzed by Diphosphino-Functionalized MCM-41 Anchored Rhodium Complex. Catal. Lett. 2012, 142, 138–142. [Google Scholar] [CrossRef]
- Modem, S.; Kankala, S.; Balaboina, R.; Thirukovela, N.S.; Jonnalagadda, S.B.; Vadde, R.; Vasam, C.S. Decarbonylation of Salicylaldehyde Activated by p-Cymene Ruthenium(II) Dimer: Implication for Catalytic Alkyne Hydrothiolation. Eur. J. Org. Chem. 2016, 2016, 4635–4642. [Google Scholar] [CrossRef]
- Peterle, M.M.; Scheide, M.R.; Silva, L.T.; Saba, S.; Rafique, J.; Braga, A.L. Copper-Catalyzed Three-Component Reaction of Oxadiazoles, Elemental Se/S and Aryl Iodides: Synthesis of Chalcogenyl (Se/S)-Oxadiazoles. ChemistrySelect 2018, 3, 13191–13196. [Google Scholar] [CrossRef]
- Maragoni, R.; Carvalho, R.E.; Macahado, M.V.; dos Santos, V.B.; Saba, S.; Botteselle, G.V.; Rafique, J. Layered Copper Hydroxide Salts as Catalyst for the “Click” Reaction and Their Application in Methyl Orange Photocatalytic Discoloration. Catalysts 2023, 13, 426. [Google Scholar] [CrossRef]
- Saba, S.; Dos Santos, C.R.; Zavarise, B.R.; Naujorks, A.A.S.; Franco, M.S.; Schneider, A.R.; Scheide, M.R.; Affeldt, R.F.; Rafique, J.; Braga, A.L. Photoinduced, Direct C(sp2)−H Bond Azo Coupling of Imidazoheteroarenes and Imidazoanilines with Aryl Diazonium Salts Catalyzed by Eosin Y. Eur. J. Chem. 2020, 26, 4461–4466. [Google Scholar] [CrossRef] [PubMed]
- Franco, M.S.; Saba, S.; Rafique, J.; Braga, A.L. KIO4-mediated Selective Hydroxymethylation/Methylenation of Imidazo-Heteroarenes: A Greener Approach. Angew. Chem. Int. Ed. 2021, 60, 18454–18460. [Google Scholar] [CrossRef]
- Doerner, C.V.; Scheide, M.R.; Nicoleti, C.R.; Durigon, D.C.; Idiarte, V.D.; Sousa, M.J.A.; Mendes, S.R.; Saba, S.; Neto, J.S.S.; Martins, G.M.; et al. Versatile Electrochemical Synthesis of Selenylbenzo[b]Furan Derivatives Through the Cyclization of 2-Alkynylphenols. Front. Chem. 2022, 20, 880099. [Google Scholar] [CrossRef]
- Locatelli, P.P.P.; Gurtat, M.; Lenz, G.F.; Marroquin, J.F.R.; Felix, J.F.; Schneider, R.; Borba, C.E. Simple borophosphate glasses for on-demand growth of self-supported copper nanoparticles in the reduction of 4-nitrophenol. J. Hazard Mater. 2021, 15, 125801. [Google Scholar] [CrossRef]
- Doerner, C.V.; Neto, J.S.S.; Cabreira, C.R.; Saba, S.; Sandjo, L.P.; Rafique, J.; Braga, A.L.; de Assis, F.R. Synthesis of 3-selanyl-isoflavones from 2-hydroxyphenyl enaminones using trichloroisocyanuric acid (TCCA): A sustainable approach. New J. Chem. 2023, 47, 5598–5602. [Google Scholar] [CrossRef]
- Tavares, C.J.; Willig, J.C.M.; Manarin, F.; Lenz, G.F.; Felix, J.F.; Botteselle, G.V.; Schneider, R. Copper nanoparticles growth on the borophosphate glass surface by bottom-up approach: A catalyst for click reactions. J. Non-Cryst. Solids 2023, 610, 122303. [Google Scholar] [CrossRef]
- Velli, L.L.; Varsamis, C.P.E.; Kamitsos, E.I.; Möncke, D.; Ehrt, D. Structural investigation of metaphosphate glasses. Phys. Chem. Glasses 2005, 46, 178–181. [Google Scholar]
- Ducel, J.F.; Videau, J.J.; Couzi, M. Structural study of borophosphate glasses by raman and infrared spectroscopy. Phys. Chem. Glas. 1993, 34, 212–218. [Google Scholar]
- Anastasopoulou, M.; Vasilopoulos, K.C.; Anagnostopoulos, D.; Koutselas, I.; Papayannis, D.K.; Karakassides, M.A. Structural and theoretical study of strontium borophosphate glasses using Raman spectroscopy and ab initio molecular orbital method. J. Phys. Chem. B 2017, 121, 4610–4619. [Google Scholar] [CrossRef] [PubMed]
- Moguš-Milanković, A.; Gajović, A.; Šantić, A.; Day, D. Structure of sodium phosphate glasses containing Al2O3 and/or Fe2O3. J. Non-Cryst. Solids 2001, 289, 204–213. [Google Scholar] [CrossRef]
- Scagliotti, M.; Villa, M.; Chiodelli, G. Short range order in the network of the borophosphate glasses: Raman results. J. Non Cryst. Solids 1987, 93, 350–360. [Google Scholar] [CrossRef]
- Hudgens, J.J.; Brow, R.K.; Tallant, D.R.; Martin, S.W. Raman Spectroscopy Study of the Structure of Lithium and Sodium Ultraphosphate Glasses. J. Non-Cryst. Solids 1998, 223, 21–31. [Google Scholar] [CrossRef]
- Lin, Y.-M.; Lu, G.-P.; Wang, G.-X.; Yi, W.-B. Acid/Phosphide-Induced Radical Route to Alkyl and Alkenyl Sulfides and Phosphonothioates from Sodium Arylsulfinates in Water. J. Org. Chem. 2017, 82, 382–389. [Google Scholar] [CrossRef]
- Ranjit, S.; Duan, Z.; Zhang, P.; Liu, X. Synthesis of Vinyl Sulfides by Copper-Catalyzed Decarboxylative C−S Cross-Coupling. Org. Lett. 2010, 12, 4134–4136. [Google Scholar] [CrossRef]
- Patel, M.; Saunthwal, R.K.; Dhaked, D.K.; Bharatam, P.V.; Verma, A.K. Nucleophilic Addition versus SNAr Study: Chemo-, Regio- and Stereoselective Hydrothiolation of Haloaryl Alkynes over S-Arylation of Aryl Halides. Asian J. Org. Chem. 2015, 4, 894–898. [Google Scholar] [CrossRef]





| Entry | Catalyst Amount (mg) | Time (min) | Temp (°C) | Yield (%) b | E:Z c |
| 1 | Fe@NaH2PO4-H3BO3 (10) | 10 | r.t. | 80 | 60:40 |
| 2 | Fe@NaH2PO4-H3BO3 (10) | 10 | 0 | 80 | 75:25 |
| 3 | Fe@NaH2PO4-H3BO3 (10) | 10 | −5 | 39 | 82:18 |
| 4 | Fe@NaH2PO4-H3BO3 (10) | 10 | 50 | 61 | 81:19 |
| 5 | Fe@NaH2PO4-H3BO3 (5) | 10 | 0 | 65 | 90:10 |
| 6 | Fe@NaH2PO4-H3BO3 (15) | 10 | 0 | 60 | 60:40 |
| 7 | Fe@NaH2PO4-H3BO3 (10) | 20 | 0 | 55 | 75:25 |
| 8 | Fe@NaH2PO4-H3BO3 (10) | 30 | 0 | 60 | 76:24 |
| 9 | Fe@NaH2PO4-H3BO3 (10) | 40 | 0 | 97 | 82:18 |
| 10 | Fe@NaH2PO4-H3BO3 (10) | 40 | r.t. | 66 | 75:25 |
| 11 | Fe@NaH2PO4-H3BO3 (10) | 40 | −5 | 44 | 88:12 |
| 12 | NaH2PO4-H3BO3 (10) | 40 | 0 | 77 | 77:23 |
| 13 d | Fe2O3 | 40 | 0 | 59 | 89:11 |
| 14 | - | 10 | 0 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Catholico, N.; Tessari, E.A.; Granja, I.J.A.; de Sousa, M.J.A.; Felix, J.F.; Manarin, F.; Godoi, M.; Rafique, J.; Schneider, R.; Saba, S.; et al. Iron-Borophosphate Glass-Catalyzed Regioselective Hydrothiolation of Alkynes under Green Conditions. Catalysts 2023, 13, 1127. https://doi.org/10.3390/catal13071127
Catholico N, Tessari EA, Granja IJA, de Sousa MJA, Felix JF, Manarin F, Godoi M, Rafique J, Schneider R, Saba S, et al. Iron-Borophosphate Glass-Catalyzed Regioselective Hydrothiolation of Alkynes under Green Conditions. Catalysts. 2023; 13(7):1127. https://doi.org/10.3390/catal13071127
Chicago/Turabian StyleCatholico, Nicoli, Eduarda A. Tessari, Isis J. A. Granja, Martinho J. A. de Sousa, Jorlandio F. Felix, Flávia Manarin, Marcelo Godoi, Jamal Rafique, Ricardo Schneider, Sumbal Saba, and et al. 2023. "Iron-Borophosphate Glass-Catalyzed Regioselective Hydrothiolation of Alkynes under Green Conditions" Catalysts 13, no. 7: 1127. https://doi.org/10.3390/catal13071127