The Role of Ion Exchange Resins for Solving Biorefinery Catalytic Processes Challenges
Abstract
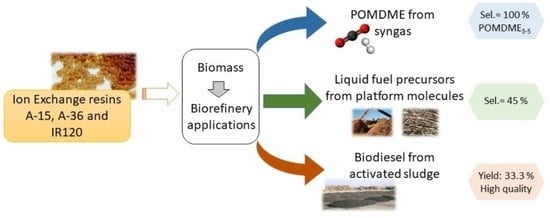
:1. Introduction
2. Results and Discussion
2.1. Dimethylether Oligomerization to Obtain POMDME (Polyoxymethylene Dimethyl Ether)
2.2. Furfural-Levulinic Acid Aldol Condensation for the Production of Liquid Fuel Additives and Precursors

2.3. Valorization of the Lipidic Fraction from Sewage Sludge for Biodiesel Production

3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bozzell, J.P.; Petersen, J.R. Technology development for the production of biobased products from biorefinery carbohydrates—The US Department of Energy’s “Top 10”. Green Chem. 2010, 12, 539–554. [Google Scholar] [CrossRef]
- Faba, L.; Díaz, E.; Ordóñez, S. Recent developments on the catalytic technologies for the transformation of biomass into biofuels: A patent survey. Renew. Sustain. Energy Rev. 2015, 51, 273–287. [Google Scholar] [CrossRef]
- Ahorsu, R.; Fedina, F.; Constantí, M. Significance and Challenges of biomass as suistable feedstock for bioenergy and biochemical production: A review. Energies 2018, 11, 3366. [Google Scholar] [CrossRef] [Green Version]
- Arias, S.; Agudelo, J.R.; Ramos, A.; Lapuerta, M. Emissions from a Euro 6 engine using polyoxymethylene dimethyl ethers: Chemical effects vs. mapping strategy. Fuel 2023, 335, 127017. [Google Scholar] [CrossRef]
- Bringue, R.; Ramirez, E.; Iborra, M.; Tejero, J.; Cunill, F. Kinetics of 1-hexanol etherification on Amberlyst 70. Chem. Eng. J. 2014, 246, 71–78. [Google Scholar] [CrossRef] [Green Version]
- De Maria, P.d.; Guajardo, N. Biocatalytic valorization of furans: Opportunities to inherently unstable substrates. ChemSusChem 2017, 10, 4123–4134. [Google Scholar] [CrossRef] [PubMed]
- Patino, Y.; Mantecón, L.G.; Polo, S.; Faba, L.; Diaz, E.; Ordonez, S. Effect of sludge features and extraction-esterification technology on the synthesis of biodiesel from secondary wastewater treatment sludges. Bioresour. Technol. 2018, 247, 209–216. [Google Scholar] [CrossRef]
- Gelbard, G. Organic synthesis by catalysis with ion-exchange resins. Ind. Eng. Chem. Res. 2005, 44, 8468–8498. [Google Scholar] [CrossRef]
- Harmer, M.A.; Sun, Q. Solid acid catalysis using ion-exchange resins. Appl. Catal. A Gen. 2001, 221, 45–62. [Google Scholar] [CrossRef]
- Săpunaru, O.V.; Sterpu, A.E.; Brînzei, M.; Pascu, S.; Koncsag, C.I. Etherification of olefins from catalytic cracking gasoline to increase its octane number. Chem. Eng. Process. 2023, 188, 109374. [Google Scholar] [CrossRef]
- Frija, L.M.T.; Afonso, C.A.M. Amberlyst®-15: A reusable heterogeneous catalyst for the dehydration of tertiary alcohols. Tetrahedron 2012, 68, 7414–7421. [Google Scholar] [CrossRef]
- Bringué, R.; Fité, C.; Iborra, M.; Tejero, J.; Cunill, F. Dehydration of 1-octanol to di-n-octyl ether in liquid phase with simultaneous water removal over ion exchange resins: Effect of working-state morphologies. Appl. Catal. A 2017, 545, 10–16. [Google Scholar] [CrossRef] [Green Version]
- Wang, R.; Wan, J.; Li, Y.; Sun, H. A further catalysis mechanism study on Amberlyst 35 resins application in alkylation desulfurization of gasoline. Chem. Eng. Sci. 2015, 137, 59–68. [Google Scholar] [CrossRef]
- Aghbashlo, M.; Tabatabaei, M.; Rastegari, H.; Ghaziaskar, H.S. Exergy-based sustainability analysis of acetins synthesis through continuous esterification of glycerol in acetic acid using Amberlyst®36 as catalyst. J. Clean. Prod. 2018, 183, 1265–1275. [Google Scholar] [CrossRef]
- Kamaruzaman, M.R.; Jiang, X.X.; Hu, X.D.; Chin, S.Y. High yield of isosorbide production from sorbitol dehydration catalysed by Amberlyst 36 under mild conditions. Chem. Eng. J. 2020, 388, 124186. [Google Scholar] [CrossRef]
- Sabou, R.; Hoelderich, W.F.; Ramprasad, D.; Weinand, R. Synthesis of 7-hydroxy-4-methylcoumarin via the Pechmann reaction with Amberlyst ion-exchange resins as catalysts. J. Catal. 2005, 232, 34–37. [Google Scholar] [CrossRef]
- Pal, R.; Sarkar, T.; Khasnobis, S. Amberlyst-15 in organic synthesis. Arkivoc 2012, 1, 570–609. [Google Scholar] [CrossRef] [Green Version]
- Delgado, J.; Vasquez Salcedo, W.N.V.; Bronzetti, G.; Moreno, V.C.; Mignot, M.; Legros, J.; Held, C.; Grénman, H.; Leveneur, S. Kinetic model assessment for the synthesis of γ-valerolactone from n-butyl levulinate and levulinic acid hydrogenation over the synergy effect of dual catalysts Ru/C and Amberlite IR-120. Chem. Eng. J. 2022, 430, 133053. [Google Scholar] [CrossRef]
- Reddy, M.; Bhojegowd, M.; Nizam, A.; Pasha, M.A. Amberlite IR-120: A Reusable Catalyst for N-Formylation of Amines with Formic Acid Using Microwaves. Chin. J. Catal. 2010, 31, 518–520. [Google Scholar]
- Baranowski, C.J.; Bahmanpour, A.M.; Kröcher, O. Catalytic synthesis of polyoxymethylene dimethyl ethers (OME): A review. Appl. Catal. B 2017, 217, 407–420. [Google Scholar] [CrossRef]
- Schmitz, N.; Burger, J.; Hasse, H. Reaction kinetics of the formation of poly(oxymethylene) dimethyl ethers from formaldehyde and methanol in aqueous solutions. Ind. Eng. Chem. Res. 2015, 54, 12553–12560. [Google Scholar] [CrossRef]
- Burger, J.; Ströfer, E.; Hasse, H. Chemical Equilibrium and Reaction Kinetics of the Heterogeneously Catalyzed Formation of Poly(oxymethylene) Dimethyl Ethers from Methylal and Trioxane. Ind. Eng. Chem. Res. 2012, 51, 12751–12761. [Google Scholar] [CrossRef]
- Zhang, J.; Shi, M.; Fang, D.; Liu, D. Reaction kinetics of the production of polyoxymethylene dimethyl ethers from methano and formaldehyde with acid cation exchange resin catalyst. React. Kinet. Mech. Catal. 2014, 113, 459–470. [Google Scholar] [CrossRef]
- Oestreich, D.; Lautenschütz, L.; Arnold, U.; Sauer, J. Reaction kinetics and equilibrium parameters for the production of oxymethylene dimethyl ethers (OME) from methanol and formaldehyde. Chem. Eng. Sci. 2017, 163, 92–104. [Google Scholar] [CrossRef]
- Ziyang, Z.; Hidajat, K.; Ray, A.K. Determination of adsorption and kinetic parameters for methyl tert-butyl ether synthesis from tert-butyl alcohol and methanol. J. Catal. 2001, 200, 209–221. [Google Scholar] [CrossRef] [Green Version]
- Peláez, R.; Marín, P.; Ordóñez, S. Effect of formaldehyde precursor and water inhibition in dimethoxymethane synthesis from methanol over acidic ion exchange resins: Mechanism and kinetics. Biofuel Bioprod. Biorefin. 2021, 15, 1696–1708. [Google Scholar] [CrossRef]
- Peláez, R.; Marín, P.; Ordóñez, S. Synthesis of poly(oxymethylene) dimethyl ethers from methylal and trioxane over acidic ion exchange resins: A kinetic study. Chem. Eng. J. 2020, 396, 125305. [Google Scholar] [CrossRef]
- He, J.; Qiang, Q.; Liu, S.M.; Song, K.; Zhous, X.W.; Guo, J.; Zhang, B.; Li, C.Z. Upgrading of biomass-derived furanic compounds into high-quality fuels involving aldol condensation strategy. Fuel 2021, 306, 121765. [Google Scholar] [CrossRef]
- Wu, L.P.; Moteki, T.; Gokhale, A.; Flaherty, D.W.; Toste, F.D. Production of fuels and chemicals from biomass: Condensation reactions and beyond. Chem 2016, 1, 32–58. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Li, Y.Q.; Qian, C.; An, L.; Wang, W.; Li, X.F.; Shao, X.Z.; Li, Z.Z. Research progress of catalysts for aldol condensation of biomass based compounds. RSC Adv. 2023, 13, 9466–9478. [Google Scholar] [CrossRef]
- Faba, L.; Cueto, J.; Díaz, E.; Ordóñez, S. From lignocellulosic biomass to chemical precursors: Simultaneous valorization of furfural and levulinic acid over mesoporous acid Catalysts. Ind. Crop. Prod. 2022, 188, 115692. [Google Scholar]
- Jian, Z.W.; Hu, D.; Zhao, Z.Y.; Yi, Z.X.; Chen, Z.; Yan, K. Mini-review on the synthesis of furfural and levulinic acid from lignocellulosic biomass. Processes 2021, 9, 1234. [Google Scholar] [CrossRef]
- Faba, L.; Díaz, E.; Ordóñez, S. Base-catalyzed condensation of levulinic acid: A new biorefinery upgrading approach. ChemCatChem 2016, 8, 1490–1494. [Google Scholar] [CrossRef]
- Cueto, J.; Korobka, V.; Faba, L.; Díaz, E.; Ordóñez, S. Aldol condensation of biomass-derived levulinic acid and furfural over acid zeolites. ACS Sustain. Chem. Eng. 2020, 8, 4371–4383. [Google Scholar] [CrossRef]
- Liang, G.; Wang, A.; Zhao, X.; Lei, N.; Zhang, T. Selective aldol condensation of bioimass-derived levulinic acid and furfural in aqueous-phase over MgO and ZnO. Green Chem. 2016, 18, 3430–3438. [Google Scholar] [CrossRef]
- Soto, R.; Oktar, N.; Fité, C.; Ramirez, E.; Bringué, R.; Tejero, J. Experimental study on the liquid-phase adsorption equilibrium of n-butanol over AmberlystTM 15 and contribution of diffusion resistances. Chem. Eng. Technol. 2021, 44, 2210–2219. [Google Scholar] [CrossRef]
- Orege, J.I.; Oderinde, O.; Kifle, G.A.; Ibikunle, A.A.; Raheem, S.A.; Ejeromedoghene, O.; Okeke, E.S.; Olukowi, O.M.; Orege, O.B.; Fagbohun, E.O.; et al. Recent advances in heterogeneous catalysis for green biodiesel production by transesterification. Energ. Conv. Manag. 2022, 258, 115406. [Google Scholar] [CrossRef]
- Go, A.W.; Sutanto, S.; Ong, L.K.; Tran-Nguyen, P.L.; Ismadji, S.; Ju, Y. Developments in in-situ (trans) esterification for biodiesel production: A critical review. Renew. Sustain. Energy Rev. 2016, 60, 284–305. [Google Scholar] [CrossRef] [Green Version]
- Carrero, A.; Vicente, G.; Rodríguez, R.; Linares, M.; del Peso, G.L. Hierarchical zeolites as catalysts for biodiesel production from Nannochloropsis microalga oil. Catal. Today 2011, 167, 148–153. [Google Scholar] [CrossRef]
- Patiño, Y.; Faba, L.; Díaz, E.; Ordóñez, S. Biodiesel production from wastewater sludge using exchange resins as heterogeneous acid catalyst: Catalyst selection and sludge pre-treatments. J. Water Process Eng. 2021, 44, 102335. [Google Scholar] [CrossRef]
- Siddiquee, M.; Rohani, S. Experimental analysis of lipid extraction and biodiesel production from wastewater sludge. Fuel Process. Technol. 2011, 92, 2241–2251. [Google Scholar] [CrossRef]
- Wu, X.; Zhu, F.; Qi, J.; Zhao, L. Biodiesel Production from Sewage Sludge by Using Alkali Catalyst Catalyze. Procedia Environ. Sci. 2016, 31, 26–30. [Google Scholar] [CrossRef] [Green Version]
- Melero, J.A.; Sánchez-Vázquez, R.; Vasiliadou, R.A.; Martínez Castillejo, F.; Bautista, L.F.; Iglesias, J.; Morales, G.; Molina, R. Municipal sewage sludge to biodiesel by simultaneous extraction and conversion of lipids. Energ. Conv. Manag. 2015, 103, 111–118. [Google Scholar] [CrossRef]
- Hatami, B.; Ebrahimi, A.A.; Ehrampoush, M.H.; Salmani, S.; Fatemeh Tamaddon, M.H.; Mokhtari, M. An efficient heterogeneous solid acid catalyst derived from sewage sludge for the catalytic transformation of sludge into biodiesel: Preparation, characterization, and arylation process modeling. J. Clean. Prod. 2022, 355, 131809. [Google Scholar] [CrossRef]
- Özbay, N.; Oktar, N.; Tapan, N.A. Esterification of free fatty acids in waste cooking oils (WCO): Role of ion-exchange resins. Fuel 2008, 87, 1789–1798. [Google Scholar] [CrossRef]
- Patiño, Y.; Faba, L.; Ordóñez, S. Effect of pretreatments and catalytic route in the quality and productivity of biodiesel obtained from secondary sludge. Biomass Bioenerg. 2021, 152, 106195. [Google Scholar] [CrossRef]




| Catalyst | Temperature (°C) | Time (h) | Trioxane Conversion (%) | POMDME Selectivity (%) |
|---|---|---|---|---|
| A-36 | 50 | 0.33 | 93.5 | 31.5 (n = 3–6) |
| SO42−/TiO2 | 80 | 1 | 89.5 | 54.8 (n = 3–8) |
| HZSM-5 | 130 | 0.75 | 85.3 | 88.5 (n = 2–8) |
| IL/SiO2 | 105 | 1 | 92 | 52.0 (n = 3–8) |
| SBET (m2/g) | Vpore (cm3/g) | Dp (nm) | Acidity (meq H+/g) | Swelling Capacity (%) | |
|---|---|---|---|---|---|
| A-15 | 80 | 0.4 | 26 | 1.04 | 10 |
| A-36 | 35 | 0.3 | 33 | 2.18 | 22 |
| IR-120 | 2 | <0.1 | 29 | 2.58 | 37 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patiño, Y.; Faba, L.; Peláez, R.; Cueto, J.; Marín, P.; Díaz, E.; Ordóñez, S. The Role of Ion Exchange Resins for Solving Biorefinery Catalytic Processes Challenges. Catalysts 2023, 13, 999. https://doi.org/10.3390/catal13060999
Patiño Y, Faba L, Peláez R, Cueto J, Marín P, Díaz E, Ordóñez S. The Role of Ion Exchange Resins for Solving Biorefinery Catalytic Processes Challenges. Catalysts. 2023; 13(6):999. https://doi.org/10.3390/catal13060999
Chicago/Turabian StylePatiño, Yolanda, Laura Faba, Raquel Peláez, Jennifer Cueto, Pablo Marín, Eva Díaz, and Salvador Ordóñez. 2023. "The Role of Ion Exchange Resins for Solving Biorefinery Catalytic Processes Challenges" Catalysts 13, no. 6: 999. https://doi.org/10.3390/catal13060999