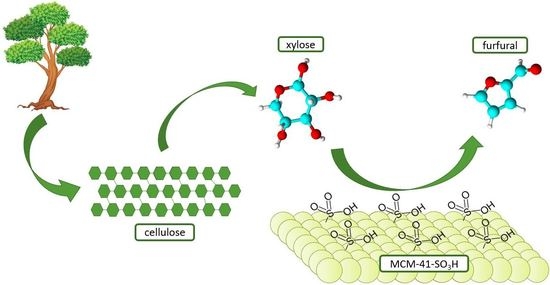
Stable Sulfonic MCM-41 Catalyst for Furfural Production from Renewable Resources in a Biphasic System
Abstract
:1. Introduction
2. Results and Discussion
2.1. ICP
2.2. N2 Physisorption
2.3. TPD-NH3
2.4. XRD
2.5. TEM
2.6. FTIR
2.7. XPS
2.8. Xylose Conversion
2.9. Hemicellulose Conversion
2.10. Catalyst Recycling
3. Materials and Methods
3.1. Synthesis of the Catalyst
3.2. Characterization Techniques
3.3. Catalytic Activity Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Haldar, D.; Purkait, M.K. A Review on the Environment-Friendly Emerging Techniques for Pretreatment of Lignocellulosic Biomass: Mechanistic Insight and Advancements. Chemosphere 2021, 264, 128523. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Ouyang, D.; Zhou, Z.; Page, S.J.; Liu, D.; Zhao, X. Lignocellulosic Biomass as Sustainable Feedstock and Materials for Power Generation and Energy Storage. J. Energy Chem. 2021, 57, 247–280. [Google Scholar] [CrossRef]
- Sella Kapu, N.; Trajano, H.L. Review of Hemicellulose Hydrolysis in Softwoods and Bamboo. Biofuels Bioprod. Bioref. 2014, 8, 857–870. [Google Scholar] [CrossRef]
- Yang, H.; Yi, N.; Zhao, S.; Qaseem, M.F.; Zheng, B.; Li, H.; Feng, J.X.; Wu, A. min Characterization of Hemicelluloses in Sugarcane (Saccharum Spp. Hybrids) Culm during Xylogenesis. Int. J. Biol. Macromol. 2020, 165, 1119–1128. [Google Scholar] [CrossRef] [PubMed]
- Mariscal, R.; Maireles-Torres, P.; Ojeda, M.; Sádaba, I.; López Granados, M. Furfural: A Renewable and Versatile Platform Molecule for the Synthesis of Chemicals and Fuels. Energy Environ. Sci. 2016, 9, 1144–1189. [Google Scholar] [CrossRef]
- Werpy, T.; Petersen, G. Top Value Added Chemicals from Biomass Volume I—Results of Screening for Potential Candidates from Sugars and Synthesis Gas; U.S. Department of Energy, Office of Scientific and Technical Information: Oak Ridge, TN, USA, 2004.
- Mizuno, N.; Misono, M. Heterogeneous Catalysis. Chem. Rev. 1998, 98, 199–216. [Google Scholar] [CrossRef]
- Sarrafi, Y.; Mehrasbi, E.; Mashalchi, S.Z. MCM-41-SO3H: An Efficient, Reusable, Heterogeneous Catalyst for the One-Pot, Three-Component Synthesis of Pyrano[3,2-b]Pyrans. Res. Chem. Intermed. 2021, 47, 1729–1741. [Google Scholar] [CrossRef]
- Beck, J.S.; Vartulli, J.C.; Roth, W.J.; Leonowicz, M.E.; Kresge, C.T.; Schmitt, K.D.; Chu, C.T.-W.; Olson, D.H.; Sheppard, E.W.; McCullen, S.B.; et al. A New Family of Mesoporous Molecular Sieves Prepared with Liquid Crystal Templates. J. Am. Chem. Soc. 1992, 114, 10834–10843. [Google Scholar] [CrossRef]
- Brezoiu, A.M.; Deaconu, M.; Nicu, I.; Vasile, E.; Mitran, R.A.; Matei, C.; Berger, D. Heteroatom Modified MCM-41-Silica Carriers for Lomefloxacin Delivery Systems. Microporous Mesoporous Mater. 2019, 275, 214–222. [Google Scholar] [CrossRef]
- Meynen, V.; Cool, P.; Vansant, E.F. Verified Syntheses of Mesoporous Materials. Microporous Mesoporous Mater. 2009, 125, 170–223. [Google Scholar] [CrossRef]
- Kresge, C.T.; Leonowiccz, M.E.; Roth, W.J.; Vartuli, J.C.; Beck, J.S. Ordered Mesoporous Molecular Sieves Synthesized by a Liquid-Crystal Template Mechanism. Nature 1992, 359, 710–712. [Google Scholar] [CrossRef]
- Safaei, S.; Mohammadpoor-Baltork, I.; Khosropour, A.R.; Moghadam, M.; Tangestaninejad, S.; Mirkhani, V. SO3H-Functionalized MCM-41 as an Efficient Catalyst for the Combinatorial Synthesis of 1H-Pyrazolo-[3,4-b]Pyridines and Spiro-Pyrazolo-[3,4-b]Pyridines. J. Iran. Chem. Soc. 2017, 14, 1583–1589. [Google Scholar] [CrossRef]
- Jeong, G.H.; Kim, E.G.; Kim, S.B.; Park, E.D.; Kim, S.W. Fabrication of Sulfonic Acid Modified Mesoporous Silica Shells and Their Catalytic Performance with Dehydration Reaction of D-Xylose into Furfural. Microporous Mesoporous Mater. 2011, 144, 134–139. [Google Scholar] [CrossRef]
- Shi, X.; Wu, Y.; Yi, H.; Rui, G.; Li, P.; Yang, M.; Wang, G. Selective Preparation of Furfural from Xylose over Sulfonic Acid Functionalized Mesoporous Sba-15 Materials. Energies 2011, 4, 669–684. [Google Scholar] [CrossRef]
- Dias, A.S.; Pillinger, M.; Valente, A.A. Dehydration of Xylose into Furfural over Micro-Mesoporous Sulfonic Acid Catalysts. J. Catal. 2005, 229, 414–423. [Google Scholar] [CrossRef]
- Zhang, J.; Zhuang, J.; Lin, L.; Liu, S.; Zhang, Z. Conversion of D-Xylose into Furfural with Mesoporous Molecular Sieve MCM-41 as Catalyst and Butanol as the Extraction Phase. Biomass Bioenergy 2012, 39, 73–77. [Google Scholar] [CrossRef]
- Bozell, J.J.; Petersen, G.R. Technology Development for the Production of Biobased Products from Biorefinery Carbohydrates—The US Department of Energy’s “Top 10” Revisited. Green Chem. 2010, 12, 539–554. [Google Scholar] [CrossRef]
- Lima, S.; Pillinger, M.; Valente, A.A. Dehydration of D-Xylose into Furfural Catalysed by Solid Acids Derived from the Layered Zeolite Nu-6(1). Catal. Commun. 2008, 9, 2144–2148. [Google Scholar] [CrossRef]
- Lima, S.; Neves, P.; Antunes, M.M.; Pillinger, M.; Ignatyev, N.; Valente, A.A. Conversion of Mono/Di/Polysaccharides into Furan Compounds Using 1-Alkyl-3-Methylimidazolium Ionic Liquids. Appl. Catal. A Gen. 2009, 363, 93–99. [Google Scholar] [CrossRef]
- Serrano-Ruiz, J.C.; Campelo, J.M.; Francavilla, M.; Romero, A.A.; Luque, R.; Menéndez-Vázquez, C.; García, A.B.; García-Suárez, E.J. Efficient Microwave-Assisted Production of Furfural from C5 Sugars in Aqueous Media Catalysed by Brönsted Acidic Ionic Liquids. Catal. Sci. Technol. 2012, 2, 1828–1832. [Google Scholar] [CrossRef]
- Marin-astorga, N.; Pecchi, G.; Reyes, P. Ordered Mesoporous Silicates Of Mcm-41 Type As Supports Of Pt Catalysts For The Enantioselective Hydrogenation Of 1-Phenyl-1, 2-Propanedione. React. Kinet. Catal. Lett. 2006, 87, 121–128. [Google Scholar] [CrossRef]
- Rostamizadeh, S.; Amani, A.M.; Mahdavinia, G.H.; Amiri, G.; Sepehrian, H. Ultrasound Promoted Rapid and Green Synthesis of 1,8-Dioxo-Octahydroxanthenes Derivatives Using Nanosized MCM-41-SO3H as a Nanoreactor, Nanocatalyst in Aqueous Media. Ultrason. Sonochem. 2010, 17, 306–309. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.L.; Klaysom, C.; Gahlaut, A.; Li, X.; Vankelecom, I.F.J. SPEEK and Functionalized Mesoporous MCM-41 Mixed Matrix Membranes for CO 2 Separations. J. Mater. Chem. 2012, 22, 20057–20064. [Google Scholar] [CrossRef]
- Wang, S.; Shi, Y.; Ma, X. Microwave Synthesis, Characterization and Transesterification Activities of Ti-MCM-41. Microporous Mesoporous Mater. 2012, 156, 22–28. [Google Scholar] [CrossRef]
- Hermida, L.; Zuhairi Abdullah, A.; Rahman Mohamed, A. Synthesis of Monoglyceride through Glycerol Esterification with Lauric Acid over Propyl Sulfonic Acid Post-Synthesis Functionalized SBA-15 Mesoporous Catalyst. Chem. Eng. J. 2011, 174, 668–676. [Google Scholar] [CrossRef]
- Sreevardhan Reddy, S.; David Raju, B.; Siva Kumar, V.; Padmasri, A.H.; Narayanan, S.; Rama Rao, K.S. Sulfonic Acid Functionalized Mesoporous SBA-15 for Selective Synthesis of 4-Phenyl-1,3-Dioxane. Catal. Commun. 2007, 8, 261–266. [Google Scholar] [CrossRef]
- Sheng, X.; Gao, J.; Han, L.; Jia, Y.; Sheng, W. One-Pot Synthesis of Tryptophols with Mesoporous MCM-41 Silica Catalyst Functionalized with Sulfonic Acid Groups. Microporous Mesoporous Mater. 2011, 143, 73–77. [Google Scholar] [CrossRef]
- Kaiprommarat, S.; Kongparakul, S.; Reubroycharoen, P.; Guan, G.; Samart, C. Highly Efficient Sulfonic MCM-41 Catalyst for Furfural Production: Furan-Based Biofuel Agent. Fuel 2016, 174, 189–196. [Google Scholar] [CrossRef]
- Karnjanakom, S.; Kongparakul, S.; Chaiya, C.; Reubroycharoen, P.; Guan, G.; Samart, C. Biodiesel Production from Hevea Brasiliensis Oil Using SO3H-MCM-41 Catalyst. J. Environ. Chem. Eng. 2016, 4, 47–55. [Google Scholar] [CrossRef]
- Bukallah, S.B.; Bumajdad, A.; Khalil, K.M.S.; Zaki, M.I. Characterization of Mesoporous VOx/MCM-41 Composite Materials Obtained via Post-Synthesis Impregnation. Appl. Surf. Sci. 2010, 256, 6179–6185. [Google Scholar] [CrossRef]
- Rathod, P.V.; Mujmule, R.B.; Chung, W.-J.; Jadhav, A.R.; Kim, H. Efficient Dehydration of Glucose, Sucrose, and Fructose to 5-Hydroxymethylfurfural Using Tri-Cationic Ionic Liquids. Catal. Lett. 2019, 149, 672–687. [Google Scholar] [CrossRef]
- Chen, K.; Yan, X.; Li, J.; Jiao, T.; Cai, C.; Zou, G.; Wang, R.; Wang, M.; Zhang, L.; Peng, Q. Preparation of Self-Assembled Composite Films Constructed by Chemically-Modified MXene and Dyes with Surface-Enhanced Raman Scattering Characterization. Nanomaterials 2019, 2, 284. [Google Scholar] [CrossRef] [Green Version]
- Hino, M.; Kurashige, M.; Matsuhashi, H.; Arata, K. The Surface Structure of Sulfated Zirconia: Studies of XPS and Thermal Analysis. Thermochim. Acta 2006, 441, 35–41. [Google Scholar] [CrossRef]
- Herrera, C.; Fuentealba, D.; Ghampson, I.T.; Sepulveda, C.; García-Fierro, J.L.; Canales, R.I.; Escalona, N. Selective Conversion of Biomass-Derived Furfural to Cyclopentanone over Carbon Nanotube-Supported Ni Catalyst in Pickering Emulsions. Catal. Commun. 2020, 144. [Google Scholar] [CrossRef]
- Moreau, C.; Durand, R.; Peyron, D.; Duhamet, J.; Rivalier, P. Selective Preparation of Furfural from Xylose over Microporous Solid Acid Catalysts. Ind. Crops Prod. 1998, 7, 95–99. [Google Scholar] [CrossRef]
- Rivalier, P.; Duhamet, J.; Moreau, C.; Durand, R. Development of a Continuous Catalytic Heterogeneous Column Reactor with Simultaneous Extraction of an Intermediate Product by an Organic Solvent Circulating in Countercurrent Manner with the Aqueous Phase. Catal. Today 1995, 24, 165–171. [Google Scholar] [CrossRef]
- Karimi, B.; Zareyee, D. Design of a Highly Efficient And-Tolerant Sulfonic Acid Based on Tunable Ordered Silica for the von Pechmann Reacton. Org. Lett. 2008, 10, 3989–3992. [Google Scholar] [CrossRef]
- Liu, C.; Wei, L.; Yin, X.; Wei, M.; Xu, J.; Jiang, J.; Wang, K. Selective Conversion of Hemicellulose into Furfural over Low-Cost Metal Salts in a γ-Valerolactone/Water Solution. Ind. Crops Prod. 2020, 147, 112248. [Google Scholar] [CrossRef]
- Agirrezabal-Telleria, I.; Gandarias, I.; Arias, P.L. Heterogeneous Acid-Catalysts for the Production of Furan-Derived Compounds (Furfural and Hydroxymethylfurfural) from Renewable Carbohydrates: A Review. Catal. Today 2014, 234, 42–58. [Google Scholar] [CrossRef]
- Madon, R.J.; Boudart, M. Experimental Criterion for the Absence of Artifacts in the Measurement of Rates of Heterogeneous Catalytic Reactions. Ind. Eng. Chem. Fundam. 1982, 21, 438–447. [Google Scholar] [CrossRef]
- Troncoso-Ortega, E.; Castillo, R.D.P.; Reyes-Contreras, P.; Castaño-Rivera, P.; Teixeira Mendonça, R.; Schiappacasse, N.; Parra, C. Effects on Lignin Redistribution in Eucalyptus Globulus Fibres Pre-Treated by Steam Explosion: A Microscale Study to Cellulose Accessibility. Biomolecules 2021, 11, 507. [Google Scholar] [CrossRef] [PubMed]











| % S | % H | % N | %C | SBET, m2g−1 | Acid Sites (mmol g−1) | |||
|---|---|---|---|---|---|---|---|---|
| Weak | Medium | Total | ||||||
| MCM41-1 | <0.1 | <2 | <2 | <0.1 | 1025 | - | - | - |
| MCM41-SO3H-1 | 14.6 | 5.3 | <2 | <0.1 | 243 | 1.4 | 0.9 | 2.3 |
| MCM41-SO3H-2 | 14.1 | 5.2 | <2 | <0.1 | 256 | 1.7 | 1.0 | 2.7 |
| Binding Energy (BE), eV | Si/O | |||||||
|---|---|---|---|---|---|---|---|---|
| Si 2p | O 1s | S 2p | Si/O | Si/S | ||||
| MCM-41 | 103.7(100) 100% | - | 533.1 100% | - | - | - | 0.18 | - |
| MCM-41-SO3H | 103.5 93% | 105.4 7% | 532.9 88% | 534.3 12% | 167.4 84% | 169.0 16% | 0.14 | 1.50 |
| Butanol/Water Ratio | XTOTAL % 2 h | YFUR % 2 h | k h−1gcat−1 | TOF s−1 |
|---|---|---|---|---|
| 1:1 | 97 | 42 | 2.6 | 0.12 |
| 1.5:1 | 100 | 46 | 3.1 | 0.15 |
| 2:1 | 92 | 41 | 2.7 | 0.11 |
| 2.5:1 | 85 | 42 | 1.9 | 0.09 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olivares, Y.; Herrera, C.; Seguel, J.; Sepúlveda, C.; Parra, C.; Pecchi, G. Stable Sulfonic MCM-41 Catalyst for Furfural Production from Renewable Resources in a Biphasic System. Catalysts 2023, 13, 1024. https://doi.org/10.3390/catal13061024
Olivares Y, Herrera C, Seguel J, Sepúlveda C, Parra C, Pecchi G. Stable Sulfonic MCM-41 Catalyst for Furfural Production from Renewable Resources in a Biphasic System. Catalysts. 2023; 13(6):1024. https://doi.org/10.3390/catal13061024
Chicago/Turabian StyleOlivares, Yasnina, Carla Herrera, Juan Seguel, Catherine Sepúlveda, Carolina Parra, and Gina Pecchi. 2023. "Stable Sulfonic MCM-41 Catalyst for Furfural Production from Renewable Resources in a Biphasic System" Catalysts 13, no. 6: 1024. https://doi.org/10.3390/catal13061024