Effect of the Preparation Method on Cu-MOR/g-C3N4 for Direct Methanol Synthesis from Methane Oxidation by Photothermal Catalysis
Abstract
:1. Introduction
2. Results
3. Discussion
3.1. Physical and Chemical Properties Characterizations
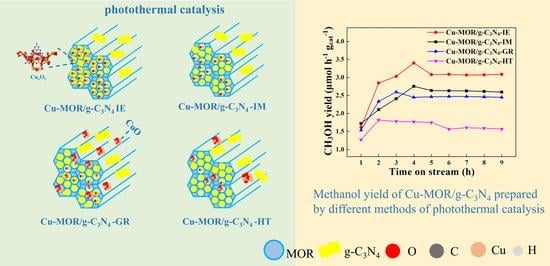
3.2. Photocatalytic Property
3.3. Thermal and Photothermal Mechanism
4. Materials and Methods
4.1. Materials
4.2. Preparation of the Catalysts
4.3. Activity Test
4.4. Catalyst Characterization
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Konno, Y.; Fujii, T.; Sato, A.; Akamine, K.; Naiki, M.; Masuda, Y.; Yamamoto, K.; Nagao, J. Key Findings of the World’s First Offshore Methane Hydrate Production Test off the Coast of Japan: Toward Future Commercial Production. Energy Fuels 2017, 31, 2607–2616. [Google Scholar] [CrossRef]
- Mahyuddin, M.H.; Shiota, Y.; Yoshizawa, K. Methane selective oxidation to methanol by metal-exchanged zeolites: A review of active sites and their reactivity. Catal. Sci. Technol. 2019, 9, 1744–1768. [Google Scholar] [CrossRef]
- McFarland, E. Unconventional Chemistry for Unconventional Natural Gas. Science 2012, 338, 340–342. [Google Scholar] [CrossRef]
- Moore, T.A. Coalbed methane: A review. Int. J. Coal Geol. 2012, 101, 36–81. [Google Scholar] [CrossRef]
- Wu, L.; Fan, W.; Wang, X.; Lin, H.; Tao, J.; Liu, Y.; Deng, J.; Jing, L.; Dai, H. Methane Oxidation over the Zeolites-Based Catalysts. Catalysts 2023, 13, 604. [Google Scholar] [CrossRef]
- Yu, C.; Shen, S. Progress in studies of natural gas conversion in China. Pet. Sci. 2008, 5, 67–72. [Google Scholar] [CrossRef]
- Han, P.; Zhang, Z.; Chen, Z.; Lin, J.; Wan, S.; Wang, Y.; Wang, S. Critical Role of Al Pair Sites in Methane Oxidation to Methanol on Cu-Exchanged Mordenite Zeolites. Catalysts 2021, 11, 751. [Google Scholar] [CrossRef]
- Tao, L.; Lee, I.-W.; Sanchez-Sanchez, M. Cu oxo nanoclusters for direct oxidation of methane to methanol: Formation, structure and catalytic performance. Catal. Sci. Technol. 2020, 10, 7124–7141. [Google Scholar] [CrossRef]
- Sirajuddin, S.; Rosenzweig, A.C. Enzymatic oxidation of methane. Biochemistry 2015, 54, 2283–2294. [Google Scholar] [CrossRef]
- Zhou, C.; Li, S.; He, S.; Zhao, Z.; Jiao, Y.; Zhang, H. Temperature-dependant active sites for methane continuous conversion to methanol over Cu-zeolite catalysts using water as the oxidant. Fuel 2022, 329, 125483. [Google Scholar] [CrossRef]
- Wulfers, M.J.; Teketel, S.; Ipek, B.; Lobo, R.F. Conversion of methane to methanol on copper-containing small-pore zeolites and zeotypes. ChemComm 2015, 51, 4447–4450. [Google Scholar] [CrossRef] [PubMed]
- Tomkins, P.; Mansouri, A.; Bozbag, S.E.; Krumeich, F.; Park, M.B.; Alayon, E.M.; Ranocchiari, M.; van Bokhoven, J.A. Isothermal Cyclic Conversion of Methane into Methanol over Copper-Exchanged Zeolite at Low Temperature. Angew. Chem. Int. Ed. 2016, 55, 5467–5471. [Google Scholar] [CrossRef] [PubMed]
- Grundner, S.; Markovits, M.A.C.; Li, G.; Tromp, M.; Pidko, E.A.; Hensen, E.J.M.; Jentys, A.; Sanchez-Sanchez, M.; Lercher, J.A. Single-site trinuclear copper oxygen clusters in mordenite for selective conversion of methane to methanol. Nat. Commun. 2015, 6, 7546. [Google Scholar] [CrossRef] [PubMed]
- Bao, J.; Yang, G.; Yoneyama, Y.; Tsubaki, N. Significant Advances in C1 Catalysis: Highly Efficient Catalysts and Catalytic Reactions. ACS Catal. 2019, 9, 3026–3053. [Google Scholar] [CrossRef]
- Koo, C.W.; Rosenzweig, A.C. Biochemistry of aerobic biological methane oxidation. Chem. Soc. Rev. 2021, 50, 3424–3436. [Google Scholar] [CrossRef]
- Eid, K.; Gamal, A.; Abdullah, A.M. Graphitic carbon nitride-based nanostructures as emergent catalysts for carbon monoxide (CO) oxidation. Green Chem. 2023, 25, 1276–1310. [Google Scholar] [CrossRef]
- Xie, P.; Ding, J.; Yao, Z.; Pu, T.; Zhang, P.; Huang, Z.; Wang, C.; Zhang, J.; Zecher-Freeman, N.; Zong, H.; et al. Oxo dicopper anchored on carbon nitride for selective oxidation of methane. Nat. Commun. 2022, 13, 1375. [Google Scholar] [CrossRef]
- Eid, K.; Sliem, M.H.; Al-Ejji, M.; Abdullah, A.M.; Harfouche, M.; Varma, R.S. Hierarchical Porous Carbon Nitride-Crumpled Nanosheet-Embedded Copper Single Atoms: An Efficient Catalyst for Carbon Monoxide Oxidation. ACS Appl. Mater. Interfaces 2022, 14, 40749–40760. [Google Scholar] [CrossRef]
- Eid, K.; Sliem, M.H.; Al-Kandari, H.; Sharaf, M.A.; Abdullah, A.M. Rational Synthesis of Porous Graphitic-like Carbon Nitride Nanotubes Codoped with Au and Pd as an Efficient Catalyst for Carbon Monoxide Oxidation. Langmuir 2019, 35, 3421–3431. [Google Scholar] [CrossRef]
- Narsimhan, K.; Iyoki, K.; Dinh, K.; Román-Leshkov, Y. Catalytic Oxidation of Methane into Methanol over Copper-Exchanged Zeolites with Oxygen at Low Temperature. ACS Cent. Sci. 2016, 2, 424–429. [Google Scholar] [CrossRef]
- Dinh, K.T.; Sullivan, M.M.; Narsimhan, K.; Serna, P.; Meyer, R.J.; Dincă, M.; Román-Leshkov, Y. Continuous Partial Oxidation of Methane to Methanol Catalyzed by Diffusion-Paired Copper Dimers in Copper-Exchanged Zeolites. J. Am. Chem. Soc. 2019, 141, 11641–11650. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Wang, Y.; Wang, C.; Xie, Z.; Guan, N.; Li, L. Water-involved methane-selective catalytic oxidation by dioxygen over copper zeolites. Chem 2021, 7, 1557–1568. [Google Scholar] [CrossRef]
- Song, C.; Wang, Z.; Yin, Z.; Xiao, D.; Ma, D. Principles and applications of photothermal catalysis. Chem. Catal. 2022, 2, 52–83. [Google Scholar] [CrossRef]
- Wei, L.; Yu, C.; Yang, K.; Fan, Q.; Ji, H. Recent advances in VOCs and CO removal via photothermal synergistic catalysis. Chin. J. Catal. 2021, 42, 1078–1095. [Google Scholar] [CrossRef]
- Mateo, D.; Cerrillo, J.L.; Durini, S.; Gascon, J. Fundamentals and applications of photo-thermal catalysis. Chem. Soc. Rev. 2021, 50, 2173–2210. [Google Scholar] [CrossRef]
- Han, L.; Mao, J.; Xie, A.-Q.; Liang, Y.; Zhu, L.; Chen, S. Synergistic enhanced solar-driven water purification and CO2 reduction via photothermal catalytic membrane distillation. Sep. Purif. Technol. 2023, 309, 123003. [Google Scholar] [CrossRef]
- Cai, M.; Wu, Z.; Li, Z.; Wang, L.; Sun, W.; Tountas, A.A.; Li, C.; Wang, S.; Feng, K.; Xu, A.-B.; et al. Greenhouse-inspired supra-photothermal CO2 catalysis. Nat. Energy 2021, 6, 807–814. [Google Scholar] [CrossRef]
- Guo, S.; Li, X.; Li, J.; Wei, B. Boosting photocatalytic hydrogen production from water by photothermally induced biphase systems. Nat. Commun. 2021, 12, 1343. [Google Scholar] [CrossRef]
- Lu, J.; Shi, Y.; Chen, Z.; Sun, X.; Yuan, H.; Guo, F.; Shi, W. Photothermal effect of carbon dots for boosted photothermal-assisted photocatalytic water/seawater splitting into hydrogen. Chem. Eng. J. 2023, 453, 139834. [Google Scholar] [CrossRef]
- Song, H.; Ye, J. Photothermal tandem catalysis for CO2 hydrogenation to methanol. Chem 2022, 8, 1181–1183. [Google Scholar] [CrossRef]
- Du, R.; Zhu, H.; Zhao, H.; Lu, H.; Dong, C.; Liu, M.; Yang, F.; Yang, J.; Wang, J.; Pan, J. Modulating photothermal properties by integration of fined Fe–Co in confined carbon layer of SiO2 nanosphere for pollutant degradation and solar water evaporation. Environ. Res. 2023, 222, 115365. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Liu, J.; Xu, Y.; Sun, Y. A review of CH4-CO2 reforming to synthesis gas over Ni-based catalysts in recent years (2010–2017). Int. J. Hydrog. Energy 2018, 43, 15030–15054. [Google Scholar] [CrossRef]
- Marinković, M.; Waisi, H.; Blagojević, S.; Zarubica, A.; Ljupković, R.; Krstić, A.; Janković, B. The effect of process parameters and catalyst support preparation methods on the catalytic efficiency in transesterification of sunflower oil over heterogeneous KI/Al2O3-based catalysts for biodiesel production. Fuel 2022, 315, 123246. [Google Scholar] [CrossRef]
- Han, M.-J.; Jiao, Y.-L.; Zhou, C.-H.; Guo, Y.-L.; Guo, Y.; Lu, G.-Z.; Wang, L.; Zhan, W.-C. Catalytic activity of Cu–SSZ-13 prepared with different methods for NH3-SCR reaction. Rare Metals 2019, 38, 210–220. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Kim, H.B.; Park, E.D. CO and CO2 Methanation over CeO2-Supported Cobalt Catalysts. Catalysts 2022, 12, 212. [Google Scholar] [CrossRef]
- Chen, Y.-Q.; Zhang, R.-X.; Ren, M.; Jin, Y.-X.; Wang, W.-J.; Feng, J.-Y.; Gao, Z.-H.; Yan, Z.-F.; Liu, Y.-M.; Huang, W.; et al. Photo-assisted thermal catalysis for methanol synthesis from methane oxidation on Cu-MOR/g-C3N4. Fuel 2023, 340, 127525. [Google Scholar] [CrossRef]
- Al-Rawi, U.A.; Sher, F.; Hazafa, A.; Bilal, M.; Lima, E.C.; Al-Shara, N.K.; Jubeen, F.; Shanshool, J. Synthesis of Zeolite supported bimetallic catalyst and application in n-hexane hydro-isomerization using supercritical CO2. J. Environ. Chem. Eng. 2021, 9, 105206. [Google Scholar] [CrossRef]
- Al-Rawi, U.A.; Sher, F.; Hazafa, A.; Rasheed, T.; Al-Shara, N.K.; Lima, E.C.; Shanshool, J. Catalytic Activity of Pt Loaded Zeolites for Hydroisomerization of n-Hexane Using Supercritical CO2. Ind. Eng. Chem. Res. 2020, 59, 22092–22106. [Google Scholar] [CrossRef]
- Tavolaro, A.; Riccio, I.I.; Tavolaro, P. Hydrothermal synthesis of zeolite composite membranes and crystals as potential vectors for drug-delivering biomaterials. Microporous Mesoporous Mater. 2013, 167, 62–70. [Google Scholar] [CrossRef]
- Zhan, H.; Huang, S.; Li, Y.; Lv, J.; Wang, S.; Ma, X. Elucidating the nature and role of Cu species in enhanced catalytic carbonylation of dimethyl ether over Cu/H-MOR. Catal. Sci. Technol. 2015, 5, 4378–4389. [Google Scholar] [CrossRef]
- Zhang, W.; Xie, J.; Hou, W.; Liu, Y.; Zhou, Y.; Wang, J. One-Pot Template-Free Synthesis of Cu–MOR Zeolite toward Efficient Catalyst Support for Aerobic Oxidation of 5-Hydroxymethylfurfural under Ambient Pressure. ACS Appl. Mater. Interfaces 2016, 8, 23122–23132. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Yuan, M.; Li, X.; Bian, S.; Mi, L.; Gao, Z.; Shi, Q.; Huang, W.; Zuo, Z. The Influence of UiO-bpy Skeleton for the Direct Methane-to-Methanol Conversion on Cu@UiO-bpy: Importance of the Encapsulation Effect. ChemCatChem 2021, 13, 4897–4902. [Google Scholar] [CrossRef]
- Chen, P.; Dong, F.; Ran, M.; Li, J. Synergistic photo-thermal catalytic NO purification of MnOx/g-C3N4: Enhanced performance and reaction mechanism. Chin. J. Catal. 2018, 39, 619–629. [Google Scholar] [CrossRef]
- Tong, Z.; Yang, D.; Shi, J.; Nan, Y.; Sun, Y.; Jiang, Z. Three-Dimensional Porous Aerogel Constructed by g-C3N4 and Graphene Oxide Nanosheets with Excellent Visible-Light Photocatalytic Performance. ACS Appl. Mater. Interfaces 2015, 7, 25693–25701. [Google Scholar] [CrossRef]
- Sainz-Vidal, A.; Balmaseda, J.; Lartundo-Rojas, L.; Reguera, E. Preparation of Cu–mordenite by ionic exchange reaction under milling: A favorable route to form the mono-(μ-oxo) dicopper active species. Microporous Mesoporous Mater. 2014, 185, 113–120. [Google Scholar] [CrossRef]
- Pappas, D.K.; Borfecchia, E.; Dyballa, M.; Pankin, I.A.; Lomachenko, K.A.; Martini, A.; Signorile, M.; Teketel, S.; Arstad, B.; Berlier, G.; et al. Methane to Methanol: Structure–Activity Relationships for Cu-CHA. J. Am. Chem. Soc. 2017, 139, 14961–14975. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, T.Y.; Lee, H.; Yi, J. Distinct activation of Cu-MOR for direct oxidation of methane to methanol. ChemComm 2017, 53, 4116–4119. [Google Scholar] [CrossRef]
- Markovits, M.A.C.; Jentys, A.; Tromp, M.; Sanchez-Sanchez, M.; Lercher, J.A. Effect of Location and Distribution of Al Sites in ZSM-5 on the Formation of Cu-Oxo Clusters Active for Direct Conversion of Methane to Methanol. Top. Catal. 2016, 59, 1554–1563. [Google Scholar] [CrossRef]
- Zuo, Z.-J.; Li, J.; Han, P.-D.; Huang, W. XPS and DFT Studies on the Autoxidation Process of Cu Sheet at Room Temperature. J. Phys. Chem. C 2014, 118, 20332–20345. [Google Scholar] [CrossRef]
- You, J.; Bao, W.; Wang, L.; Yan, A.; Guo, R. Preparation, visible light-driven photocatalytic activity, and mechanism of multiphase CdS/C3N4 inorganic-organic hybrid heterojunction. J. Alloys Compd. 2021, 866, 158921. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, L.; Wang, W. Direct functionalization of methane into ethanol over copper modified polymeric carbon nitride via photocatalysis. Nat. Commun. 2019, 10, 506. [Google Scholar] [CrossRef] [PubMed]
- Groothaert, M.H.; Smeets, P.J.; Sels, B.F.; Jacobs, P.A.; Schoonheydt, R.A. Selective Oxidation of Methane by the Bis(μ-oxo)dicopper Core Stabilized on ZSM-5 and Mordenite Zeolites. J. Am. Chem. Soc. 2005, 127, 1394–1395. [Google Scholar] [CrossRef] [PubMed]
- Tan, F.; Liu, M.; Li, K.; Wang, Y.; Wang, J.; Guo, X.; Zhang, G.; Song, C. Facile synthesis of size-controlled MIL-100(Fe) with excellent adsorption capacity for methylene blue. Chem. Eng. J. 2015, 281, 360–367. [Google Scholar] [CrossRef]
- Férey, G.; Serre, C.; Mellot-Draznieks, C.; Millange, F.; Surblé, S.; Dutour, J.; Margiolaki, I. A Hybrid Solid with Giant Pores Prepared by a Combination of Targeted Chemistry, Simulation, and Powder Diffraction. Angew. Chem. Int. Ed. 2004, 43, 6296–6301. [Google Scholar] [CrossRef]
- Yang, L.; Ren, X.; Zhang, Y.; Chen, Z.; Wan, J. One-step synthesis of a heterogeneous catalyst: Cu+-decorated triazine-based g-C3N4 nanosheet formation and catalytic mechanism. J. Environ. Chem. Eng. 2021, 9, 105558. [Google Scholar] [CrossRef]
- Wu, X.; Zhao, Q.; Zhang, J.; Li, S.; Liu, H.; Liu, K.; Li, Y.; Kong, D.; Sun, H.; Wu, M. 0D carbon dots intercalated Z-scheme CuO/g-C3N4 heterojunction with dual charge transfer pathways for synergetic visible-light-driven photo-Fenton-like catalysis. J. Colloid Interface Sci. 2023, 634, 972–982. [Google Scholar] [CrossRef]
- Mohamed, R.M.; Ismail, A.A. Triblock copolymer-assisted synthesis of Z-scheme porous g-C3N4 based photocatalysts with promoted visible-light-driven performance. Ceram. Int. 2020, 46, 28903–28913. [Google Scholar] [CrossRef]
- Wang, R.; Gu, L.; Zhou, J.; Liu, X.; Teng, F.; Li, C.; Shen, Y.; Yuan, Y. Quasi-Polymeric Metal–Organic Framework UiO-66/g-C3N4 Heterojunctions for Enhanced Photocatalytic Hydrogen Evolution under Visible Light Irradiation. Adv. Mater. Interfaces 2015, 2, 1500037. [Google Scholar] [CrossRef]
- Dai, Z.; Lian, J.; Sun, Y.; Li, L.; Zhang, H.; Hu, N.; Ding, D. Fabrication of g-C3N4/Sn3O4/Ni electrode for highly efficient photoelectrocatalytic reduction of U(VI). Chem. Eng. J. 2022, 433, 133766. [Google Scholar] [CrossRef]
- Karimi-Nazarabad, M.; Ahmadzadeh, H.; Goharshadi, E.K. Porous perovskite-lanthanum cobaltite as an efficient cocatalyst in photoelectrocatalytic water oxidation by bismuth doped g-C3N4. Sol. Energy 2021, 227, 426–437. [Google Scholar] [CrossRef]
- Demchenko, A.P.; Tomin, V.I.; Chou, P.-T. Breaking the Kasha Rule for More Efficient Photochemistry. Chem. Rev. 2017, 117, 13353–13381. [Google Scholar] [CrossRef] [PubMed]
- Beznis, N.V.; Weckhuysen, B.M.; Bitter, J.H. Partial Oxidation of Methane Over Co-ZSM-5: Tuning the Oxygenate Selectivity by Altering the Preparation Route. Catal. Letters 2010, 136, 52–56. [Google Scholar] [CrossRef]
- Le, H.V.; Parishan, S.; Sagaltchik, A.; Göbel, C.; Schlesiger, C.; Malzer, W.; Trunschke, A.; Schomäcker, R.; Thomas, A. Solid-State Ion-Exchanged Cu/Mordenite Catalysts for the Direct Conversion of Methane to Methanol. ACS Catal. 2017, 7, 1403–1412. [Google Scholar] [CrossRef]
- He, P.; Li, Y.; Cai, K.; Xiong, X.; Lv, J.; Wang, Y.; Huang, S.; Ma, X. Nano-Assembled Mordenite Zeolite with Tunable Morphology for Carbonylation of Dimethyl Ether. ACS Appl. Nano Mater. 2020, 3, 6460–6468. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, M.; Huang, Y.; Shi, X.; Zhang, Y.; Huang, T.; Cao, J.; Ho, W.; Lee, S.C. Self-assembly synthesis of boron-doped graphitic carbon nitride hollow tubes for enhanced photocatalytic NOx removal under visible light. Appl. Catal. B 2018, 239, 352–361. [Google Scholar] [CrossRef]










| Methanol Yield (μmol h−1 gcat−1) | Percentage | ||
|---|---|---|---|
| Photo-Thermal | Thermal | ||
| Cu-MOR/g-C3N4-IE | 3.09 | 2.45 | 26.1% |
| Cu-MOR/g-C3N4-IM | 2.59 | 2.10 | 23.3% |
| Cu-MOR/g-C3N4-GR | 2.45 | 2.00 | 22.5% |
| Cu-MOR/g-C3N4-HT | 1.57 | 1.50 | 4.67% |
| Catalyst | Cu Content (wt%) | Cu/Al |
|---|---|---|
| Cu-MOR/g-C3N4-IE | 0.69 | 0.23 |
| Cu-MOR/g-C3N4-IM | 1.03 | 0.32 |
| Cu-MOR/g-C3N4-GR | 1.40 | 0.76 |
| Cu-MOR/g-C3N4-HT | 2.19 | 1.11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hao, J.-C.; Zhang, R.-X.; Ren, M.; Zhao, J.-X.; Gao, Z.-H.; Liu, L.; Zhang, Z.-X.; Zuo, Z.-J. Effect of the Preparation Method on Cu-MOR/g-C3N4 for Direct Methanol Synthesis from Methane Oxidation by Photothermal Catalysis. Catalysts 2023, 13, 868. https://doi.org/10.3390/catal13050868
Hao J-C, Zhang R-X, Ren M, Zhao J-X, Gao Z-H, Liu L, Zhang Z-X, Zuo Z-J. Effect of the Preparation Method on Cu-MOR/g-C3N4 for Direct Methanol Synthesis from Methane Oxidation by Photothermal Catalysis. Catalysts. 2023; 13(5):868. https://doi.org/10.3390/catal13050868
Chicago/Turabian StyleHao, Jun-Cai, Rui-Xin Zhang, Miao Ren, Jia-Xuan Zhao, Zhi-Hua Gao, Lei Liu, Zhu-Xia Zhang, and Zhi-Jun Zuo. 2023. "Effect of the Preparation Method on Cu-MOR/g-C3N4 for Direct Methanol Synthesis from Methane Oxidation by Photothermal Catalysis" Catalysts 13, no. 5: 868. https://doi.org/10.3390/catal13050868