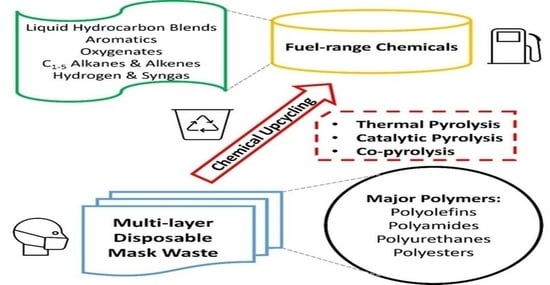
Preparing Fuel-Range Chemicals via the Direct and Selective Pyrolysis of Disposable Mask Waste for Sustainable Environment
Abstract
:1. Introduction
2. Direct Characterizations of Disposable Masks
2.1. Determination of Disposable Mask Constituents
2.2. Identification of Disposable Mask Degradation Temperatures
3. Fuel-Range Liquids via Selective Mask Pyrolysis
3.1. Liquid Hydrocarbon Blends
3.2. Aromatic Hydrocarbons
3.3. Oxygenated Liquids
4. Fuel-Range Gases via Selective Mask Pyrolysis
4.1. C1–5 Alkanes and Alkenes
4.2. Hydrogen and Syngas
5. Comparison of Various Pyrolytic Processes
6. Conclusions and Outlooks
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ellis, L.D.; Rorrer, N.A.; Sullivan, K.P.; Otto, M.; McGeehan, J.E.; Román-Leshkov, Y.; Wierckx, N.; Beckham, G.T. Chemical and biological catalysis for plastics recycling and upcycling. Nat. Catal. 2021, 4, 539–556. [Google Scholar] [CrossRef]
- Jehanno, C.; Alty, J.W.; Roosen, M.; De Meester, S.; Dove, A.P.; Chen, E.Y.-X.; Leibfarth, F.A.; Sardon, H. Critical advances and future opportunities in upcycling commodity polymers. Nature 2022, 603, 803–814. [Google Scholar] [CrossRef] [PubMed]
- Thiounn, T.; Smith, R.C. Advances and approaches for chemical recycling of plastic waste. J. Polym. Sci. 2020, 58, 1347–1364. [Google Scholar] [CrossRef] [Green Version]
- Zhang, F.; Wang, F.; Wei, X.; Yang, Y.; Xu, S.; Deng, D.; Wang, Y.-Z. From trash to treasure: Chemical recycling and upcycling of commodity plastic waste to fuels, high-valued chemicals and advanced materials. J. Energy Chem. 2022, 69, 369–388. [Google Scholar] [CrossRef]
- Chen, H.; Wan, K.; Zhang, Y.; Wang, Y. Waste to Wealth: Chemical Recycling and Chemical Upcycling of Waste Plastics for a Great Future. ChemSusChem 2021, 14, 4123–4136. [Google Scholar] [CrossRef]
- Ragaert, K.; Delva, L.; Van Geem, K. Mechanical and chemical recycling of solid plastic waste. Waste Manag. 2017, 69, 24–58. [Google Scholar] [CrossRef]
- Lange, J.-P. Managing Plastic Waste─Sorting, Recycling, Disposal, and Product Redesign. ACS Sustain. Chem. Eng. 2021, 9, 15722–15738. [Google Scholar] [CrossRef]
- Schyns, Z.O.G.; Shaver, M.P. Mechanical Recycling of Packaging Plastics: A Review. Macromol. Rapid Commun. 2021, 42, 2000415. [Google Scholar] [CrossRef]
- Nikiema, J.; Asiedu, Z. A review of the cost and effectiveness of solutions to address plastic pollution. Environ. Sci. Pollut. Res. Int. 2022, 29, 24547–24573. [Google Scholar] [CrossRef]
- Dissanayake, L.; Jayakody, L.N. Engineering Microbes to Bio-Upcycle Polyethylene Terephthalate. Front. Bioeng. Biotechnol. 2021, 9, 656465. [Google Scholar] [CrossRef]
- Tiso, T.; Narancic, T.; Wei, R.; Pollet, E.; Beagan, N.; Schröder, K.; Honak, A.; Jiang, M.; Kenny, S.T.; Wierckx, N.; et al. Towards bio-upcycling of polyethylene terephthalate. Metab. Eng. 2021, 66, 167–178. [Google Scholar] [CrossRef]
- Lu, H.; Diaz, D.J.; Czarnecki, N.J.; Zhu, C.; Kim, W.; Shroff, R.; Acosta, D.J.; Alexander, B.R.; Cole, H.O.; Zhang, Y.; et al. Machine learning-aided engineering of hydrolases for PET depolymerization. Nature 2022, 604, 662–667. [Google Scholar] [CrossRef]
- Kim, H.T.; Kim, J.K.; Cha, H.G.; Kang, M.J.; Lee, H.S.; Khang, T.U.; Yun, E.J.; Lee, D.-H.; Song, B.K.; Park, S.J.; et al. Biological Valorization of Poly(ethylene terephthalate) Monomers for Upcycling Waste PET. ACS Sustain. Chem. Eng. 2019, 7, 19396–19406. [Google Scholar] [CrossRef]
- Almeida, D.; de Fátima Marques, M. Thermal and catalytic pyrolysis of plastic waste. Polímeros 2016, 26, 44–51. [Google Scholar] [CrossRef] [Green Version]
- Vollmer, I.; Jenks, M.J.F.; Roelands, M.C.P.; White, R.J.; van Harmelen, T.; de Wild, P.; van der Laan, G.P.; Meirer, F.; Keurentjes, J.T.F.; Weckhuysen, B.M. Beyond Mechanical Recycling: Giving New Life to Plastic Waste. Angew. Chem. Int. Ed. 2020, 59, 15402–15423. [Google Scholar] [CrossRef] [Green Version]
- Tan, T.; Wang, W.; Zhang, K.; Zhan, Z.; Deng, W.; Zhang, Q.; Wang, Y. Upcycling Plastic Wastes into Value-Added Products by Heterogeneous Catalysis. ChemSusChem 2022, 15, e202200522. [Google Scholar] [CrossRef]
- Yansaneh, O.Y.; Zein, S.H. Latest Advances in Waste Plastic Pyrolytic Catalysis. Processes 2022, 10, 683. [Google Scholar] [CrossRef]
- Zhou, H.; Wang, Y.; Ren, Y.; Li, Z.; Kong, X.; Shao, M.; Duan, H. Plastic Waste Valorization by Leveraging Multidisciplinary Catalytic Technologies. ACS Catal. 2022, 12, 9307–9324. [Google Scholar] [CrossRef]
- Zhang, F.; Zeng, M.; Yappert, R.D.; Sun, J.; Lee, Y.-H.; LaPointe, A.M.; Peters, B.; Abu-Omar, M.M.; Scott, S.L. Polyethylene upcycling to long-chain alkylaromatics by tandem hydrogenolysis/aromatization. Science 2020, 370, 437–441. [Google Scholar] [CrossRef]
- Hou, Q.; Zhen, M.; Qian, H.; Nie, Y.; Bai, X.; Xia, T.; Laiq Ur Rehman, M.; Li, Q.; Ju, M. Upcycling and catalytic degradation of plastic wastes. Cell Rep. Phys. Sci. 2021, 2, 100514. [Google Scholar] [CrossRef]
- Fadillah, G.; Fatimah, I.; Sahroni, I.; Musawwa, M.M.; Mahlia, T.M.I.; Muraza, O. Recent Progress in Low-Cost Catalysts for Pyrolysis of Plastic Waste to Fuels. Catalysts 2021, 11, 837. [Google Scholar] [CrossRef]
- Zhou, N.; Dai, L.; Lv, Y.; Li, H.; Deng, W.; Guo, F.; Chen, P.; Lei, H.; Ruan, R. Catalytic pyrolysis of plastic wastes in a continuous microwave assisted pyrolysis system for fuel production. Chem. Eng. J. 2021, 418, 129412. [Google Scholar] [CrossRef]
- Tian, X.; Zeng, Z.; Liu, Z.; Dai, L.; Xu, J.; Yang, X.; Yue, L.; Liu, Y.; Ruan, R.; Wang, Y. Conversion of low-density polyethylene into monocyclic aromatic hydrocarbons by catalytic pyrolysis: Comparison of HZSM-5, Hβ, HY and MCM-41. J. Clean. Prod. 2022, 358, 131989. [Google Scholar] [CrossRef]
- Matuszewska, A.; Owczuk, M.; Biernat, K. Current Trends in Waste Plastics’ Liquefaction into Fuel Fraction: A Review. Energies 2022, 15, 2719. [Google Scholar] [CrossRef]
- Ayodeji, S.O.; Oni, T.O. Thermal pyrolysis production of liquid fuel from a mixture of polyethylene terephthalate and polystyrene. Heat Transf.—Asian Res. 2019, 48, 1648–1662. [Google Scholar] [CrossRef] [Green Version]
- Al-Salem, S.M.; Antelava, A.; Constantinou, A.; Manos, G.; Dutta, A. A review on thermal and catalytic pyrolysis of plastic solid waste (PSW). J. Environ. Manag. 2017, 197, 177–198. [Google Scholar] [CrossRef]
- Miandad, R.; Rehan, M.; Barakat, M.A.; Aburiazaiza, A.S.; Khan, H.; Ismail, I.M.I.; Dhavamani, J.; Gardy, J.; Hassanpour, A.; Nizami, A.-S. Catalytic Pyrolysis of Plastic Waste: Moving Toward Pyrolysis Based Biorefineries. Front. Energy Res. 2019, 7, 27. [Google Scholar] [CrossRef] [Green Version]
- Praveen Kumar, K.; Srinivas, S. Catalytic Co-pyrolysis of Biomass and Plastics (Polypropylene and Polystyrene) Using Spent FCC Catalyst. Energy Fuels 2020, 34, 460–473. [Google Scholar] [CrossRef]
- Fekhar, B.; Miskolczi, N.; Bhaskar, T.; Kumar, J.; Dhyani, V. Co-pyrolysis of biomass and plastic wastes: Investigation of apparent kinetic parameters and stability of pyrolysis oils. IOP Conf. Ser. Earth Environ. Sci. 2018, 154, 012022. [Google Scholar] [CrossRef]
- Uzoejinwa, B.B.; He, X.; Wang, S.; El-Fatah Abomohra, A.; Hu, Y.; Wang, Q. Co-pyrolysis of biomass and waste plastics as a thermochemical conversion technology for high-grade biofuel production: Recent progress and future directions elsewhere worldwide. Energy Convers. Manag. 2018, 163, 468–492. [Google Scholar] [CrossRef]
- Ke, L.; Wang, Y.; Wu, Q.; Zhou, N.; Dai, L.; Tian, X.; Huang, W.; Peng, Y.; Xu, J.; Zou, R.; et al. Pressurized ex-situ catalytic co-pyrolysis of polyethylene and lignin: Efficient BTEX production and process mechanism analysis. Chem. Eng. J. 2022, 431, 134122. [Google Scholar] [CrossRef]
- Sun, S.; Yuan, Y.; Chen, R.; Xu, X.; Zhang, D. Kinetic, thermodynamic and chemical reaction analyses of typical surgical face mask waste pyrolysis. Therm. Sci. Eng. Prog. 2021, 26, 101135. [Google Scholar] [CrossRef]
- Papari, S.; Bamdad, H.; Berruti, F. Pyrolytic Conversion of Plastic Waste to Value-Added Products and Fuels: A Review. Materials 2021, 14, 2586. [Google Scholar] [CrossRef]
- Singh, R.K.; Ruj, B.; Sadhukhan, A.K.; Gupta, P. Impact of fast and slow pyrolysis on the degradation of mixed plastic waste: Product yield analysis and their characterization. J. Energy Inst. 2019, 92, 1647–1657. [Google Scholar] [CrossRef]
- Cavalcante, J.; Hardian, R.; Szekely, G. Antipathogenic upcycling of face mask waste into separation materials using green solvents. Sustain. Mater. Technol. 2022, 32, e00448. [Google Scholar] [CrossRef]
- Akhbarizadeh, R.; Dobaradaran, S.; Nabipour, I.; Tangestani, M.; Abedi, D.; Javanfekr, F.; Jeddi, F.; Zendehboodi, A. Abandoned Covid-19 personal protective equipment along the Bushehr shores, the Persian Gulf: An emerging source of secondary microplastics in coastlines. Mar. Pollut. Bull. 2021, 168, 112386. [Google Scholar] [CrossRef]
- Prata, J.C.; Silva, A.L.P.; Walker, T.R.; Duarte, A.C.; Rocha-Santos, T. COVID-19 Pandemic Repercussions on the Use and Management of Plastics. Environ. Sci. Technol. 2020, 54, 7760–7765. [Google Scholar] [CrossRef]
- Yu, R.; Wen, X.; Liu, J.; Wang, Y.; Chen, X.; Wenelska, K.; Mijowska, E.; Tang, T. A green and high-yield route to recycle waste masks into CNTs/Ni hybrids via catalytic carbonization and their application for superior microwave absorption. Appl. Catal. B Environ. 2021, 298, 120544. [Google Scholar] [CrossRef]
- Sun, X.; Liu, Z.; Shi, L.; Liu, Q. Pyrolysis of COVID-19 disposable masks and catalytic cracking of the volatiles. J. Anal. Appl. Pyrolysis 2022, 163, 105481. [Google Scholar] [CrossRef]
- Jung, S.; Lee, S.; Dou, X.; Kwon, E.E. Valorization of disposable COVID-19 mask through the thermo-chemical process. Chem. Eng. J. 2021, 405, 126658. [Google Scholar] [CrossRef]
- Muhyuddin, M.; Filippi, J.; Zoia, L.; Bonizzoni, S.; Lorenzi, R.; Berretti, E.; Capozzoli, L.; Bellini, M.; Ferrara, C.; Lavacchi, A.; et al. Waste Face Surgical Mask Transformation into Crude Oil and Nanostructured Electrocatalysts for Fuel Cells and Electrolyzers. ChemSusChem 2022, 15, e202102351. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Yuan, X.; Sun, Z.; Suvarna, M.; Hu, X.; Wang, X.; Ok, Y.S. Pyrolysis of waste surgical masks into liquid fuel and its life-cycle assessment. Bioresour. Technol. 2022, 346, 126582. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Zhu, X.; Deng, J.; Gong, K.; Zhu, X. High-value utilization of mask and heavy fraction of bio-oil: From hazardous waste to biochar, bio-oil, and graphene films. J. Hazard. Mater. 2021, 420, 126570. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Cao, L.; Li, W.; Du, X.; Lin, Z.; Zhang, P. Carbon Nanotube prepared by catalytic pyrolysis as the electrode for supercapacitors from polypropylene wasted face masks. Ionics 2022, 28, 3489–3500. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Jiang, Z.; Yu, H.; Tang, T. Catalytic pyrolysis of polypropylene to synthesize carbon nanotubes and hydrogen through a two-stage process. Polym. Degrad. Stab. 2011, 96, 1711–1719. [Google Scholar] [CrossRef]
- Pandey, S.; Karakoti, M.; Surana, K.; Dhapola, P.S.; SanthiBhushan, B.; Ganguly, S.; Singh, P.K.; Abbas, A.; Srivastava, A.; Sahoo, N.G. Graphene nanosheets derived from plastic waste for the application of DSSCs and supercapacitors. Sci. Rep. 2021, 11, 3916. [Google Scholar] [CrossRef]
- Gong, J.; Liu, J.; Wen, X.; Jiang, Z.; Chen, X.; Mijowska, E.; Tang, T. Upcycling Waste Polypropylene into Graphene Flakes on Organically Modified Montmorillonite. Ind. Eng. Chem. Res. 2014, 53, 4173–4181. [Google Scholar] [CrossRef]
- Robertson, M.; Güillen Obando, A.; Emery, J.; Qiang, Z. Multifunctional Carbon Fibers from Chemical Upcycling of Mask Waste. ACS Omega 2022, 7, 12278–12287. [Google Scholar] [CrossRef]
- Fadare, O.O.; Okoffo, E.D. Covid-19 face masks: A potential source of microplastic fibers in the environment. Sci. Total Environ. 2020, 737, 140279. [Google Scholar] [CrossRef]
- Saliu, F.; Veronelli, M.; Raguso, C.; Barana, D.; Galli, P.; Lasagni, M. The release process of microfibers: From surgical face masks into the marine environment. Environ. Adv. 2021, 4, 100042. [Google Scholar] [CrossRef]
- Long, Y.; Hu, T.; Liu, L.; Chen, R.; Guo, Q.; Yang, L.; Cheng, Y.; Huang, J.; Du, L. Effectiveness of N95 respirators versus surgical masks against influenza: A systematic review and meta-analysis. J. Evid.-Based Med. 2020, 13, 93–101. [Google Scholar] [CrossRef]
- Cheng, Y.; Ma, N.; Witt, C.; Rapp, S.; Wild, P.S.; Andreae, M.O.; Pöschl, U.; Su, H. Face masks effectively limit the probability of SARS-CoV-2 transmission. Science 2021, 372, 1439–1443. [Google Scholar] [CrossRef]
- Park, C.; Choi, H.; Andrew Lin, K.-Y.; Kwon, E.E.; Lee, J. COVID-19 mask waste to energy via thermochemical pathway: Effect of Co-Feeding food waste. Energy 2021, 230, 120876. [Google Scholar] [CrossRef]
- Szefer, E.M.; Majka, T.M.; Pielichowski, K. Characterization and Combustion Behavior of Single-Use Masks Used during COVID-19 Pandemic. Materials 2021, 14, 3501. [Google Scholar] [CrossRef]
- Jung, M.R.; Horgen, F.D.; Orski, S.V.; Rodriguez, C.V.; Beers, K.L.; Balazs, G.H.; Jones, T.T.; Work, T.M.; Brignac, K.C.; Royer, S.-J.; et al. Validation of ATR FT-IR to identify polymers of plastic marine debris, including those ingested by marine organisms. Mar. Pollut. Bull. 2018, 127, 704–716. [Google Scholar] [CrossRef]
- Liu, J.; Wang, B.; Zhang, L.W.; Zhu, L.; Luo, T.Y. Thermal Aging Behavior of Ethylene Propylene Diene Monomer (EPDM) Rubber. Appl. Mech. Mater. 2015, 727–728, 47–50. [Google Scholar] [CrossRef]
- Ossman, M.E.; Mansour, M.S.; Fattah, M.A.; Taha, N.; Kiros, Y. Peanut shells and talc powder for removal of hexavalent chromium from aqueous solutions. Bulg. Chem. Commun. 2014, 46, 629–639. Available online: http://bcc.bas.bg/BCC_Volumes/Volume_46_Number_3_Supplement_2014/BCC-3380-Ossman-46-3-629-639.pdf (accessed on 1 March 2023).
- Lee, K.-H.; Kim, K.-W.; Pesapane, A.; Kim, H.-Y.; Rabolt, J.F. Polarized FT-IR Study of Macroscopically Oriented Electrospun Nylon-6 Nanofibers. Macromolecules 2008, 41, 1494–1498. [Google Scholar] [CrossRef]
- Wu, Q.; Liu, X.; Berglund, L.A. FT-IR spectroscopic study of hydrogen bonding in PA6/clay nanocomposites. Polymer 2002, 43, 2445–2449. [Google Scholar] [CrossRef]
- Pant, H.R.; Baek, W.; Nam, K.-T.; Jeong, I.-S.; Barakat, N.A.M.; Kim, H.Y. Effect of lactic acid on polymer crystallization chain conformation and fiber morphology in an electrospun nylon-6 mat. Polymer 2011, 52, 4851–4856. [Google Scholar] [CrossRef]
- Corrêa, H.L.; Corrêa, D.G. Polymer Applications for Medical Care in the COVID-19 Pandemic Crisis: Will We Still Speak Ill of These Materials? Front. Mater. 2020, 7, 283. [Google Scholar] [CrossRef]
- Ali, L.; Kuttiyathil, M.S.; Altarawneh, M. Catalytic upgrading of the polymeric constituents in Covid-19 masks. J. Environ. Chem. Eng. 2022, 10, 106978. [Google Scholar] [CrossRef]
- Chen, R.; Zhang, D.; Xu, X.; Yuan, Y. Pyrolysis characteristics, kinetics, thermodynamics and volatile products of waste medical surgical mask rope by thermogravimetry and online thermogravimetry-Fourier transform infrared-mass spectrometry analysis. Fuel 2021, 295, 120632. [Google Scholar] [CrossRef]
- Lee, S.B.; Lee, J.; Tsang, Y.F.; Kim, Y.-M.; Jae, J.; Jung, S.-C.; Park, Y.-K. Production of value-added aromatics from wasted COVID-19 mask via catalytic pyrolysis. Environ. Pollut. 2021, 283, 117060. [Google Scholar] [CrossRef]
- Wang, C.; Zou, R.; Lei, H.; Qian, M.; Lin, X.; Mateo, W.; Wang, L.; Zhang, X.; Ruan, R. Biochar-advanced thermocatalytic salvaging of the waste disposable mask with the production of hydrogen and mono-aromatic hydrocarbons. J. Hazard. Mater. 2022, 426, 128080. [Google Scholar] [CrossRef]
- Skrzyniarz, M.; Sajdak, M.; Zajemska, M.; Iwaszko, J.; Biniek-Poskart, A.; Skibiński, A.; Morel, S.; Niegodajew, P. Plastic Waste Management towards Energy Recovery during the COVID-19 Pandemic: The Example of Protective Face Mask Pyrolysis. Energies 2022, 15, 2629. [Google Scholar] [CrossRef]
- Salema, A.A.; Mohd Zaifullizan, Y.; Wong, W.H. Pyrolysis and combustion kinetics of disposable surgical face mask produced during COVID-19 pandemic. Energy Sources A Recovery Util. Environ. Eff. 2022, 44, 566–576. [Google Scholar] [CrossRef]
- Yousef, S.; Eimontas, J.; Striūgas, N.; Abdelnaby, M.A. Pyrolysis kinetic behaviour and TG-FTIR-GC–MS analysis of Coronavirus Face Masks. J. Anal. Appl. Pyrolysis 2021, 156, 105118. [Google Scholar] [CrossRef]
- Chheda, J.N.; Huber, G.W.; Dumesic, J.A. Liquid-Phase Catalytic Processing of Biomass-Derived Oxygenated Hydrocarbons to Fuels and Chemicals. Angew. Chem. Int. Ed. 2007, 46, 7164–7183. [Google Scholar] [CrossRef]
- Huber, G.W.; Iborra, S.; Corma, A. Synthesis of Transportation Fuels from Biomass: Chemistry, Catalysts, and Engineering. Chem. Rev. 2006, 106, 4044–4098. [Google Scholar] [CrossRef] [Green Version]
- Samanta, S.; Srivastava, R. Catalytic conversion of CO2 to chemicals and fuels: The collective thermocatalytic/photocatalytic/electrocatalytic approach with graphitic carbon nitride. Mater. Adv. 2020, 1, 1506–1545. [Google Scholar] [CrossRef]
- Karmacharya, M.; Kumar, S.; Gulenko, O.; Cho, Y.-K. Advances in Facemasks during the COVID-19 Pandemic Era. ACS Appl. Bio Mater. 2021, 4, 3891–3908. [Google Scholar] [CrossRef]
- Yousef, S.; Eimontas, J.; Stasiulaitiene, I.; Zakarauskas, K.; Striūgas, N. Pyrolysis of all layers of surgical mask waste as a mixture and its life-cycle assessment. Sustain. Prod. Consum. 2022, 32, 519–531. [Google Scholar] [CrossRef]
- Yousef, S.; Eimontas, J.; Striūgas, N.; Abdelnaby, M.A. A new strategy for butanol extraction from COVID-19 mask using catalytic pyrolysis process over ZSM-5 zeolite catalyst and its kinetic behavior. Thermochim. Acta 2022, 711, 179198. [Google Scholar] [CrossRef]
- Xin, H.; Hu, X.; Cai, C.; Wang, H.; Zhu, C.; Li, S.; Xiu, Z.; Zhang, X.; Liu, Q.; Ma, L. Catalytic Production of Oxygenated and Hydrocarbon Chemicals From Cellulose Hydrogenolysis in Aqueous Phase. Front. Chem. 2020, 8, 333. [Google Scholar] [CrossRef]
- Bai, J.; Zhang, Y.; Zhang, X.; Wang, C.; Ma, L. Synthesis of High-Density Components of Jet Fuel from Lignin-Derived Aromatics via Alkylation and Subsequent Hydrodeoxygenation. ACS Sustain. Chem. Eng. 2021, 9, 7112–7119. [Google Scholar] [CrossRef]
- Gutiérrez-Antonio, C.; Gómez-Castro, F.I.; de Lira-Flores, J.A.; Hernández, S. A review on the production processes of renewable jet fuel. Renew. Sustain. Energy Rev. 2017, 79, 709–729. [Google Scholar] [CrossRef]
- Thompson, B.; Machas, M.; Nielsen, D.R. Creating pathways towards aromatic building blocks and fine chemicals. Curr. Opin. Biotechnol. 2015, 36, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Alherech, M.; Omolabake, S.; Holland, C.M.; Klinger, G.E.; Hegg, E.L.; Stahl, S.S. From Lignin to Valuable Aromatic Chemicals: Lignin Depolymerization and Monomer Separation via Centrifugal Partition Chromatography. ACS Cent. Sci. 2021, 7, 1831–1837. [Google Scholar] [CrossRef]
- Jiménez-Cruz, F.; Laredo, G.C. Molecular size evaluation of linear and branched paraffins from the gasoline pool by DFT quantum chemical calculations. Fuel 2004, 83, 2183–2188. [Google Scholar] [CrossRef]
- Lin, W.; Song, Y.; Han, L.; Yang, X.; Liu, J.; Peng, B. Dehydrogenative aromatization of 1-octene over multifunctional Ni/ZSM-5-P-Fe catalyst. Fuel 2021, 299, 120890. [Google Scholar] [CrossRef]
- Balat, M.; Balat, H. Recent trends in global production and utilization of bio-ethanol fuel. Appl. Energy 2009, 86, 2273–2282. [Google Scholar] [CrossRef]
- Yilmaz, N.; Vigil, F.M.; Benalil, K.; Davis, S.M.; Calva, A. Effect of biodiesel–butanol fuel blends on emissions and performance characteristics of a diesel engine. Fuel 2014, 135, 46–50. [Google Scholar] [CrossRef]
- Fadzillah, D.M.; Kamarudin, S.K.; Zainoodin, M.A.; Masdar, M.S. Critical challenges in the system development of direct alcohol fuel cells as portable power supplies: An overview. Int. J. Hydrog. Energy 2019, 44, 3031–3054. [Google Scholar] [CrossRef]
- Knothe, G. Improving biodiesel fuel properties by modifying fatty ester composition. Energy Environ. Sci. 2009, 2, 759–766. [Google Scholar] [CrossRef]
- Alptekin, E.; Canakci, M. Characterization of the key fuel properties of methyl ester–diesel fuel blends. Fuel 2009, 88, 75–80. [Google Scholar] [CrossRef]
- Semelsberger, T.A.; Borup, R.L.; Greene, H.L. Dimethyl ether (DME) as an alternative fuel. J. Power Sources 2006, 156, 497–511. [Google Scholar] [CrossRef]
- Japar, S.M.; Wallington, T.J.; Richert, J.F.O.; Ball, J.C. The atmospheric chemistry of Oxygenated fuel additives: T-Butyl alcohol, dimethyl ether, and methylt-butyl ether. Int. J. Chem. Kinet. 1990, 22, 1257–1269. [Google Scholar] [CrossRef]
- Ibrahim, A. Investigating the effect of using diethyl ether as a fuel additive on diesel engine performance and combustion. Appl. Therm. Eng. 2016, 107, 853–862. [Google Scholar] [CrossRef]
- Zhou, C.-H.; Xia, X.; Lin, C.-X.; Tong, D.-S.; Beltramini, J. Catalytic conversion of lignocellulosic biomass to fine chemicals and fuels. Chem. Soc. Rev. 2011, 40, 5588–5617. [Google Scholar] [CrossRef]
- Mascal, M. Chemicals from biobutanol: Technologies and markets. Biofuels Bioprod. Biorefin. 2012, 6, 483–493. [Google Scholar] [CrossRef]
- He, X.; Wen, X.; Wu, K.; Liu, H. Sustainable synthesis of vinyl methyl ether from biomass-derived ethylene glycol dimethyl ether over solid base catalysts. Green Chem. 2021, 23, 6625–6631. [Google Scholar] [CrossRef]
- Hou, Y.; Feng, Z.; He, Y.; Gao, Q.; Ni, L.; Su, M.; Ren, H.; Liu, Z.; Hu, W. Co-pyrolysis characteristics and synergistic interaction of bamboo residues and disposable face mask. Renew. Energy 2022, 194, 415–425. [Google Scholar] [CrossRef]
- Wang, J.; Zhong, Z.; Ding, K.; Li, M.; Hao, N.; Meng, X.; Ruan, R.; Ragauskas, A.J. Catalytic fast co-pyrolysis of bamboo sawdust and waste tire using a tandem reactor with cascade bubbling fluidized bed and fixed bed system. Energy Convers. Manag. 2019, 180, 60–71. [Google Scholar] [CrossRef]
- Lee, N.; Lin, K.-Y.A.; Lee, J. Carbon dioxide-mediated thermochemical conversion of banner waste using cobalt oxide catalyst as a strategy for plastic waste treatment. Environ. Res. 2022, 213, 113560. [Google Scholar] [CrossRef]
- Dorado, C.; Mullen, C.A.; Boateng, A.A. H-ZSM5 Catalyzed Co-Pyrolysis of Biomass and Plastics. ACS Sustain. Chem. Eng. 2014, 2, 301–311. [Google Scholar] [CrossRef]
- Kumar Mishra, R.; Mohanty, K. Co-pyrolysis of waste biomass and waste plastics (polystyrene and waste nitrile gloves) into renewable fuel and value-added chemicals. Carbon Resour. Convers. 2020, 3, 145–155. [Google Scholar] [CrossRef]
- Ansari, K.B.; Hassan, S.Z.; Bhoi, R.; Ahmad, E. Co-pyrolysis of biomass and plastic wastes: A review on reactants synergy, catalyst impact, process parameter, hydrocarbon fuel potential, COVID-19. J. Environ. Chem. Eng. 2021, 9, 106436. [Google Scholar] [CrossRef]
- Jiraroj, D.; Chaipurimat, A.; Kerdsa, N.; Hannongbua, S.; Tungasmita, D.N. Catalytic cracking of polypropylene using aluminosilicate catalysts. J. Anal. Appl. Pyrolysis 2016, 120, 529–539. [Google Scholar] [CrossRef]
- Obalı, Z.; Sezgi, N.A.; Doğu, T. Catalytic degradation of polypropylene over alumina loaded mesoporous catalysts. Chem. Eng. J. 2012, 207–208, 421–425. [Google Scholar] [CrossRef]
- Abbas-Abadi, M.S.; Ureel, Y.; Eschenbacher, A.; Vermeire, F.H.; Varghese, R.J.; Oenema, J.; Stefanidis, G.D.; Van Geem, K.M. Challenges and opportunities of light olefin production via thermal and catalytic pyrolysis of end-of-life polyolefins: Towards full recyclability. Prog. Energy Combust. Sci. 2023, 96, 101046. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, Y.; Shan, R.; Gu, J.; Huhe, T.; Ling, X.; Yuan, H.; Chen, Y. High-yield H2 production from polypropylene through pyrolysis-catalytic reforming over activated carbon based nickel catalyst. J. Clean. Prod. 2022, 352, 131566. [Google Scholar] [CrossRef]
- Czernik, S.; French, R.J. Production of Hydrogen from Plastics by Pyrolysis and Catalytic Steam Reform. Energy Fuels 2006, 20, 754–758. [Google Scholar] [CrossRef]
- Cortazar, M.; Gao, N.; Quan, C.; Suarez, M.A.; Lopez, G.; Orozco, S.; Santamaria, L.; Amutio, M.; Olazar, M. Analysis of hydrogen production potential from waste plastics by pyrolysis and in line oxidative steam reforming. Fuel Process. Technol. 2022, 225, 107044. [Google Scholar] [CrossRef]
- Aminu, I.; Nahil, M.A.; Williams, P.T. Hydrogen from Waste Plastics by Two-Stage Pyrolysis/Low-Temperature Plasma Catalytic Processing. Energy Fuels 2020, 34, 11679–11689. [Google Scholar] [CrossRef]
- Foffi, R.; Savuto, E.; Stante, M.; Mancini, R.; Gallucci, K. Study of Energy Valorization of Disposable Masks via Thermochemical Processes: Devolatilization Tests and Simulation Approach. Energies 2022, 15, 2103. [Google Scholar] [CrossRef]
- Harussani, M.M.; Sapuan, S.M.; Rashid, U.; Khalina, A.; Ilyas, R.A. Pyrolysis of polypropylene plastic waste into carbonaceous char: Priority of plastic waste management amidst COVID-19 pandemic. Sci. Total Environ. 2022, 803, 149911. [Google Scholar] [CrossRef]










| No. | Possible Products | Formula | Molecular Weight | Family |
|---|---|---|---|---|
| 1 | methane | CH4 | 16 | Alkanes |
| 2 | water | H2O | 18 | inorganic substances |
| 3 | ethylene | C2H4 | 28 | alkenes |
| 4 | carbon monoxide | CO | 28 | inorganic substances |
| 5 | propylene | C3H6 | 42 | alkenes |
| 6 | carbon dioxide | CO2 | 44 | inorganic substances |
| 7 | acetaldehyde | C2H4O | 44 | aldehydes |
| 8 | butene | C4H8 | 56 | alkenes |
| 9 | acetone | C3H6O | 58 | ketones |
| 10 | 1,3-pentadiene | C5H8 | 68 | alkenes |
| 11 | 2-amylene | C5H10 | 70 | alkenes |
| 12 | 1,2-dimethyl cyclopropane | C5H10 | 70 | naphthenic hydrocarbons |
| 13 | methacrylaldehyde | C4H6O | 70 | aldehydes |
| 14 | pentane | C5H12 | 72 | alkanes |
| 15 | 2,4-hexadiene | C6H10 | 82 | alkenes |
| 16 | 2-methyl-1,3-pentadiene | C6H10 | 82 | alkenes |
| 17 | 2-hexene | C6H12 | 84 | alkenes |
| Catalyst | HZSM-5 a | HBeta a | HBeta b | HY a | Al-MCM-41 a |
|---|---|---|---|---|---|
| BTEX | 21.07 | 49.41 | 1.12 | 35.21 | 11.53 |
| Other mono-aromatic hydrocarbons other than BTEX (OMAHs) | 5.53 | 21.48 | 0 | 28.12 | 1.26 |
| Polycyclic aromatic hydrocarbons (PAHs) | 0 | 7.04 | 0 | 2.51 | 0 |
| n-Parafins | 7.49 | 1.4 | 0 | 2.15 | 8.27 |
| i-Parafins | 0 | 5.25 | 0 | 23.4 | 0 |
| n-Olefins | 16.44 | 0.38 | 8.19 | 0 | 6.05 |
| i-Olefins | 31.68 | 7.56 | 78.51 | 5.63 | 41.18 |
| Naphthenes | 16.15 | 7.11 | 3.2 | 1.44 | 16.66 |
| Others c | 1.65 | 0.37 | 8.98 | 1.54 | 15.05 |
| Key Pyrolytic Factors | Thermal Pyrolysis | Catalytic Pyrolysis | Co-Pyrolysis |
|---|---|---|---|
| Main Reactor Type | Fixed bed reactor | Fixed bed reactor | Fixed bed reactor |
| Main Reactor Stage | 1 or 2 | 1 or 2 | 1 |
| Main Reaction Temperature Range (°C) | 400–900 | 200–900 | 500–900 |
| or fixed temperature | or fixed temperature | or fixed temperature | |
| Main Reaction Heating Rate (°C/min) | 5–30 | 5–30 | 5–30 |
| Main Reaction Carrier Gas | N2 or CO2 | N2 or CO2 | N2 or CO2 |
| Main Mediator | N/A | Solid catalysts | Biomass |
| (e.g., zeolites, metal oxides, etc) | (e.g., biooil, biowaste, etc) | ||
| Main Products | Liquid blends | BTEX | C6+ hydrocarbons |
| Alkanes/Alkenes | OMAHs | Oxygenates | |
| Ethers | PAHs | Amines | |
| C1–4 gases | Alkenes | Alkanes/Alkenes | |
| Oxygenates | |||
| C1–4 gases | |||
| H2/Syngas |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, X.; Chang, C.-R. Preparing Fuel-Range Chemicals via the Direct and Selective Pyrolysis of Disposable Mask Waste for Sustainable Environment. Catalysts 2023, 13, 743. https://doi.org/10.3390/catal13040743
Gao X, Chang C-R. Preparing Fuel-Range Chemicals via the Direct and Selective Pyrolysis of Disposable Mask Waste for Sustainable Environment. Catalysts. 2023; 13(4):743. https://doi.org/10.3390/catal13040743
Chicago/Turabian StyleGao, Xin, and Chun-Ran Chang. 2023. "Preparing Fuel-Range Chemicals via the Direct and Selective Pyrolysis of Disposable Mask Waste for Sustainable Environment" Catalysts 13, no. 4: 743. https://doi.org/10.3390/catal13040743