Biogasoline Obtained Using Catalytic Pyrolysis of Desmodesmus sp. Microalgae: Comparison between Dry Biomass and n-Hexane Extract
Abstract
:1. Introduction
2. Results and Discussion
2.1. Thermogravimetry
2.2. Fourier Transform Infrared Spectroscopy (FTIR)
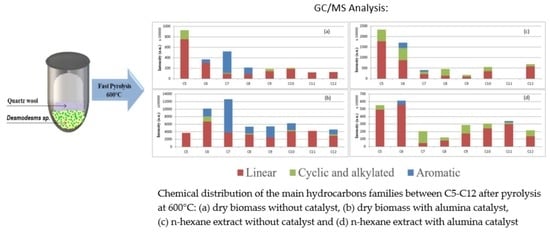
2.3. Pyrolysis of the Microalgae Biomass and n-Hexane Extract
3. Materials and Methods
3.1. Reagents
3.2. Cultivation of the Microalgae
3.3. Thermogravimetric Analyses of Biomass
3.4. Elementary Characterisation (CHN)
3.5. Fourier Transform Infrared (FTIR) Spectroscopy of n-Hexane Extract
3.6. Pyrolysis and Product Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chiaramonti, D.; Prussi, M.; Buffi, M.; Tacconi, D. Sustainable bio kerosene: Process routes and industrial demonstration activities in aviation biofuels. Appl. Energy 2014, 136, 767–774. [Google Scholar] [CrossRef]
- Hassan, S.N.; Sani, Y.M.; Abdul Aziz, A.R.; Sulaiman, N.M.N.; Daud, W.M.A.W. Biogasoline: An out-of-the-box solution to the food-for-fuel and land-use competitions. Energy Convers. Manag. 2015, 89, 349–367. [Google Scholar] [CrossRef] [Green Version]
- Kargbo, H.; Harris, J.; Phan, A. “Drop-in” fuel production from biomass: Critical review on techno-economic feasibility and sustainability. Renew. Sust. Energy Rev. 2021, 135, 110168. [Google Scholar] [CrossRef]
- Hari, T.; Yaako, Z.; Binitha, N. Aviation biofuel from renewable resources: Routes, opportunities and challenges. Renew. Sust. Energy Rev. 2015, 42, 1234–1244. [Google Scholar] [CrossRef]
- Yang, J.; Xin, Z.; Quan, H.; Corscadden, K.; Niu, H. An overview on performance characteristics of bio-jet fuels. Fuel 2019, 237, 916–936. [Google Scholar] [CrossRef]
- Fan, L.; Zhang, H.; Li, J.; Wang, Y.; Zhou, W. Algal biorefinery to value-added products by using combined processes based on thermochemical conversion: A review. Algal Res. 2020, 47, 101819. [Google Scholar] [CrossRef]
- Dourado, M.; Fonseca, N.; Sales, E.A.; Frety, R. Fast pyrolysis of microalgae Nannochloropsis Oculata for production of green diesel fraction. Int. J. Eng. Res. Appl. 2020, 10, 54–68. [Google Scholar]
- Gegg, P.; Ison, S. the market development of aviation biofuel: Drivers and constraints. J. Air Transp. Manag. 2014, 39, 34–40. [Google Scholar] [CrossRef] [Green Version]
- Du, Z.; Mohr, M.; Ma, X.; Cheng, Y.; Lin, X.; Liu, Y.; Zhou, W.; Chen, P.; Ruan, R. Hydrothermal pretreatment of microalgae for production of pyrolytic bio-oil with a low nitrogen content. Bioresour. Technol. 2012, 120, 13–18. [Google Scholar] [CrossRef]
- Li, F.; Srivatsa, S.; Bhattacharya, S. A review on catalytic pyrolysis of microalgae to high-quality bio-oil with low oxygenous and nitrogenous compounds. Renew. Sust. Energy Rev. 2019, 108, 481–497. [Google Scholar] [CrossRef]
- Yu, J.; Maliutina, K.; Tahmasebi, A. A review on the production of nitrogen-containing compounds from microalgal biomass via pyrolysis. Bioresour. Technol. 2018, 270, 689–701. [Google Scholar] [CrossRef] [PubMed]
- Prado, G.; Rao, Y.; Klerk, A. Nitrogen removal from oil: A review. Energy Fuels 2017, 31, 14–36. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, Y.; Hua, D.; Li, C.; Harold, M.; Wanga, J.; Yang, M. Thermochemical conversion of low-lipid microalgae for the production of liquid fuels: Challenges and opportunities. Rsc. Adv. 2015, 5, 18673–18701. [Google Scholar] [CrossRef]
- Fonseca, N.; Oliveira, V.; Frety, R.; Sales, E.A. Thermal and Catalytic Fast Pyrolysis of Oily Extracts of Microalgae: Production of Biokerosene. J. Braz. Chem. Soc. 2021, 32, 811–822. [Google Scholar] [CrossRef]
- Barros, R.; Raposo, S.; Morais, E.G.; Rodrigues, B.; Afonso, V.; Gonçalves, P.; Marques, J.; Cerqueira, P.R.; Varela, J.; Teixeira, M.R.; et al. Biogas Production from Microalgal Biomass Produced in the Tertiary Treatment of Urban Wastewater: Assessment of Seasonal Variations. Energies 2022, 15, 5713. [Google Scholar] [CrossRef]
- Zhao, G.; Wang, X.; Hong, Y.; Liu, X.; Wang, Q.; Zhai, Q.; Zhang, H. Attached cultivation of microalgae on rational carriers for swine wastewater treatment and biomass harvesting. Bioresour. Technol. 2022, 351, 27014. [Google Scholar] [CrossRef]
- Zieliński, M.; Dębowski, M.; Kazimierowicz, J. Outflow from a Biogas Plant as a Medium for Microalgae Biomass Cultivation—Pilot Scale Study and Technical Concept of a Large-Scale Installation. Energies 2022, 15, 2912. [Google Scholar] [CrossRef]
- Do, J.; Jo, S.; Kim, I.; Na, H.; Lee, J.; Kim, H.; Yoon, H. A Feasibility Study of Wastewater Treatment Using Domestic Microalgae and Analysis of Biomass for Potential Applications. Water 2019, 11, 2294. [Google Scholar] [CrossRef] [Green Version]
- Li, G.; Dong, R.; Fu, N.; Zhou, Y.; Li, D.; Chen, X.D. Characterization of pyrolysis products obtained from Desmodesmus sp. cultivated in anaerobic digested effluents (DADE). Int. J. Food Eng. 2015, 11, 825–832. [Google Scholar] [CrossRef]
- Li, G.; Shunan, X.; Fang, J.; Yuguang, Z.; Zhigang, H. Thermal cracking products and bio-oil production from microalgae Desmodesmus sp. Int. J. Agric. Biol. Eng. 2017, 10, 198–206. [Google Scholar] [CrossRef] [Green Version]
- Torri, C.; Fabbri, D.; Garcia-Alba, L.; Brilman, D. Upgrading of oils derived from hydrothermal treatment of microalgae by catalytic cracking over H-ZSM-5: A comparative Py-GC-MS study. J. Anal. Appl. Pyrolysis 2013, 101, 28–34. [Google Scholar] [CrossRef]
- Dos Anjos, J.R.; Gonzalez, W.; Lam, Y.; Fréty, R. Catalytic decomposition of vegetable oil. Appl. Catal. 1983, 5, 299–308. [Google Scholar] [CrossRef]
- Billaud, F.; Guitard, Y.; Tran Minh, K.; Zahraa, O.; Lozano, P.; Pioch, D. Kinetic studies of catalytic cracking of octanoic acid. J. Mol. Catal. A Chem. 2003, 192, 281–288. [Google Scholar] [CrossRef]
- Santos, M.; Arias, S.; Padilha, J.; Carneiro, M.; Sales, E.A.; Pacheco, J.; Fréty, R. Catalytic cracking of palmitic and oleic acids pre-adsorbed on γ-alumina. Catal. Today 2020, 344, 234–239. [Google Scholar] [CrossRef]
- Fonseca, N.; Pereira, A.; Fréty, R.; Sales, E.A. Fast catalytic pyrolysis of dilaurin in the presence of sodium carbonate alone or combined with alumina. Catalysts 2019, 9, 993. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Z.; Liu, P.; Wang, S.; Ma, S.; Cao, J. Combustion characteristics and kinetics of five tropic oil algal strains using thermogravimetric analysis. J. Therm. Anal. Calorim. 2018, 131, 1919–1931. [Google Scholar] [CrossRef]
- Santana Junior, J.A.; Carvalho, W.S.; Ataíde, C.H. Catalytic effect of ZSM-5 zeolite and HY-340 niobic acid on the pyrolysis of industrial kraft lignins. Ind. Crops Prod. 2018, 111, 126–132. [Google Scholar] [CrossRef]
- Kosinov, N.; Uslamin, E.A.; Coumans, F.J.A.G.; Wijpkema, A.S.G.; Rohling, R.Y.; Hensen, E.J.M. Structure and Evolution of Confined Carbon Species during Methane Dehydroaromatization over Mo/ZSM-5. ACS Catal. 2018, 8, 8459–8467. [Google Scholar] [CrossRef] [Green Version]
- Marcilla, A.; Gómez-Siurana, A.; Gomis, C.; Chápuli, E.; Catalá, C.; Valdés, J. Characterization of microalgal species through TGA/FTIR analysis: Application to Nannochloropsis sp. Thermochim. Acta 2009, 484, 41–47. [Google Scholar] [CrossRef]
- Miglio, R.; Palmery, L.; Salvalaggio, M.; Carnelli, L.; Capuano, F.; Borrelli, R. Microalgae triacylglycerols content by FTIR spectroscopy. J. Appl. Phycol. 2013, 1621–1631. [Google Scholar] [CrossRef]
- Maher, K.; Bressler, D. Pyrolysis of triglyceride materials for the production of renewable fuels and chemicals. Bioresour. Technol. 2007, 98, 2351–2368. [Google Scholar] [CrossRef] [PubMed]
- Asomaning, J.; Mussone, P.; Bressler, C. Thermal deoxygenation and pyrolysis of mono unsaturated fatty acids. J. Anal. Appl. Pyrol. 2014, 105, 1–7. [Google Scholar] [CrossRef]
- Vonghia, E.; Boocock, D.; Konar, S.; Leung, A. Pathways for the deoxygenation of triglycerides to aliphatic hydrocarbons over activated alumina. Energy Fuels 1995, 9, 1090–1096. [Google Scholar] [CrossRef]
- Leung, A.; Boocock, D.; Konar, S. Pathway for the catalytic conversion of carboxylic acids to hydrocarbons over activated alumina. Energy Fuels 1995, 9, 913–920. [Google Scholar] [CrossRef]
- Zhao, H.; Zhong, Z.; Li, Z.; Wang, W. Research on catalytic pyrolysis of algae based on Py-GC/MS. R. Soc. Open Sci. 2019, 6, 191307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chagas, B.M.E.; Dorado, C.; Serapiglia, M.J.; Mullen, C.A.; Boateng, A.A.; Melo, M.A.F.; Ataíde, C.H. Catalytic pyrolysis-GC/MS of Spirulina: Evaluation of a highly proteinaceous biomass source for production of fuels and chemicals. Fuel 2016, 179, 124–134. [Google Scholar] [CrossRef]
- Guibet, J.-C.; Faure-Birchem, E.; Lévy, R.H. Carburants et Moteurs: Technologies, Energie, Environnement. Tome, 2nd ed.; Technip: Paris, France, 1997; ISBN 2-7108-0704-1. [Google Scholar]
- Engineering ToolBox—Fuels—Higher and Lower Calorific Values. Available online: https://www.engineeringtoolbox.com/fuels-higher-calorific-values-d_169.html (accessed on 6 November 2022).
- Hydrogen Tools—Lower and Higher Heating Values of Fuels. Available online: https://h2tools.org/hyarc/calculator-tools/lower-and-higher-heating-values-fuels (accessed on 6 November 2022).






| Atmosphere | Event I up to 200 °C (%) | Event II 200–450 °C (%) | Event III above 450 °C (%) | Ash + Char (%) | % C | % H | % N | % Others |
|---|---|---|---|---|---|---|---|---|
| Synthetic air | 8 | 48 | 33 | 11 | 43.0 | 13.8 | 6.4 | 36.8 |
| Nitrogen | 10 | 22 | 17 | 55 |
| Alumina | % C5–C12 Products in the Whole Pyrogram | % C5–C12 Hydrocarbons in the Whole Pyrogram | % Nitrogenated Compounds in the C5–C12 Fraction | % Oxygenated Compounds in the C5–C12 Fraction | % Other C5–C12 Products in the Whole Pyrogram | |
|---|---|---|---|---|---|---|
| Dry biomass | no | 18.5 | 13.7 | 0.5 | 0.7 | 4.8 |
| Dry biomass | yes | 48.7 | 40.4 | 6.4 | 0.2 | 8.3 |
| Extract | no | 24.9 | 24.4 | b.d.l. * | b.d.l. * | 0.5 |
| Extract | yes | 44.6 | 33.5 | b.d.l. * | 2.6 | 11.1 |
| Sample (Desmodesmus sp.) | Alumina Catalyst | Type of Molecules (% Area) | ||
|---|---|---|---|---|
| Linear | Cyclic and Alkylated | Aromatics | ||
| Dry biomass | no | 9.4 | 1.3 | 3.1 |
| Dry biomass | yes | 25.3 | 2.4 | 14.6 |
| Extract | no | 15.5 | 8.5 | 1.3 |
| Extract | yes | 20.7 | 4.9 | 2.8 |
| Sample (Desmodesmus sp.) | Alumina Catalyst | Mean Low Heating Value (MJ/kg) |
|---|---|---|
| Dry biomass | no | 44 |
| Dry biomass | yes | 43 |
| Extract | no | 44 |
| Extract | yes | 43 |
| Type of Hydrocarbon | Standard Deviation (%) |
|---|---|
| Alkane | 7.3 |
| Alkene | 2.7 |
| Aromatic | 2.1 |
| General average | 5.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fonseca, N.; Fréty, R.; Sales, E.A. Biogasoline Obtained Using Catalytic Pyrolysis of Desmodesmus sp. Microalgae: Comparison between Dry Biomass and n-Hexane Extract. Catalysts 2022, 12, 1517. https://doi.org/10.3390/catal12121517
Fonseca N, Fréty R, Sales EA. Biogasoline Obtained Using Catalytic Pyrolysis of Desmodesmus sp. Microalgae: Comparison between Dry Biomass and n-Hexane Extract. Catalysts. 2022; 12(12):1517. https://doi.org/10.3390/catal12121517
Chicago/Turabian StyleFonseca, Noyala, Roger Fréty, and Emerson Andrade Sales. 2022. "Biogasoline Obtained Using Catalytic Pyrolysis of Desmodesmus sp. Microalgae: Comparison between Dry Biomass and n-Hexane Extract" Catalysts 12, no. 12: 1517. https://doi.org/10.3390/catal12121517