Highly Effectual Photocatalytic Remediation of Tetracycline under the Broad Spectrum of Sunlight by Novel BiVO4/Sb2S3 Nanocomposite
Abstract
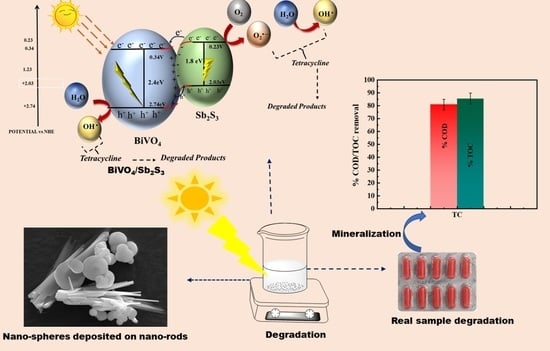
:1. Introduction
2. Results and Discussions
2.1. X-ray Photo Electron Study (XPS)
2.2. X-ray Diffraction Analysis (XRD)
2.3. Photoluminescence Spectra (PL)
2.4. Surface Area Analyses
2.5. Absorption Studies
2.6. Scanning Electron Microscopy (SEM) Analysis
2.7. Energy Dispersive Spectroscopy (EDS) and Elemental Mapping
2.8. Photocatalytic Studies
2.8.1. Impact of the Amount of Catalyst Loading
2.8.2. Effect of Light Sources
2.8.3. Impact of pH
2.8.4. Scavenger Studies
2.8.5. Mineralization Experiment
2.9. GC–MS Analysis
| Photocatalyst | Concentration of Pollutant | Reaction Time (min) | Light Source | Rate Constant k | Degradation Efficiency (%) | Ref. |
|---|---|---|---|---|---|---|
| BiVO4/BiOI | 10 ppm | 60 | Visible light | 0.0133 min−1 | 69.57 | [55] |
| BiVO4/Bi2SiO5 | 10 ppm | 120 | Visible light | 0.0072 min−1 | 72.2 | [56] |
| ZnInS4/Sb2S3 | 10 ppm | 140 | Visible light | 0.009 min−1 | 85.36 | [57] |
| Ag2WO4/Sb2S3 | 10 ppm | 180 | Visible light | 0.0041 min−1 | 53.06 | [25] |
| BiOBr/g-C3N4/carbon nanofibers | - | 120 | Visible light | 0.015 min−1 | 86.1 | [9] |
| BVSBS | 10 ppm | 120 | Sunlight | 0.01557 min−1 | 88.7 | Current work |
2.10. Photocatalytic Degradation Mechanism
3. Materials and Methods
3.1. Synthesis Protocol of BiVO4
3.2. Synthesis Method of the BiVO4/Sb2S3 Hybrid
3.3. Methods of Characterization
3.4. Photocatalytic Elimination of TC
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singla, S.; Sharma, S.; Basu, S. MoS2/WO3 Heterojunction with the Intensified Photocatalytic Performance for Decomposition of Organic Pollutants under the Broad Array of Solar Light. J. Clean. Prod. 2021, 324, 129290. [Google Scholar] [CrossRef]
- Singla, S.; Sharma, S.; Basu, S.; Shetti, N.P.; Reddy, K.R. Graphene/Graphitic Carbon Nitride-Based Ternary Nanohybrids: Synthesis Methods, Properties, and Applications for Photocatalytic Hydrogen Production. FlatChem 2020, 24, 100200. [Google Scholar] [CrossRef]
- Singla, S.; Sharma, S.; Basu, S.; Shetti, N.P.; Aminabhavi, T.M. Photocatalytic Water Splitting Hydrogen Production via Environmental Benign Carbon Based Nanomaterials. Int. J. Hydrog. Energy 2021, 46, 33696–33717. [Google Scholar] [CrossRef]
- Pichel, N.; Vivar, M.; Fuentes, M. The Problem of Drinking Water Access: A Review of Disinfection Technologies with an Emphasis on Solar Treatment Methods. Chemosphere 2019, 218, 1014–1030. [Google Scholar] [CrossRef] [PubMed]
- Singla, S.; Singh, P.; Basu, S.; Devi, P. BiVO4/MoSe2 Photocatalyst for the Photocatalytic Abatement of Tetracycline and Photoelectrocatalytic Water Splitting. Mater. Chem. Phys. 2023, 295, 127111. [Google Scholar] [CrossRef]
- Yu, H.; Ge, D.; Liu, Y.; Lu, Y.; Wang, X.; Huo, M.; Qin, W. One-Pot Synthesis of BiOCl Microflowers Co-Modified with Mn and Oxygen Vacancies for Enhanced Photocatalytic Degradation of Tetracycline under Visible Light. Sep. Purif. Technol. 2020, 251, 117414. [Google Scholar] [CrossRef]
- Fiaz, A.; Zhu, D.; Sun, J. Environmental Fate of Tetracycline Antibiotics: Degradation Pathway Mechanisms, Challenges, and Perspectives. Environ. Sci. Eur. 2021, 33, 64. [Google Scholar] [CrossRef]
- Guo, J.; Jiang, L.; Liang, J.; Xu, W.; Yu, H.; Zhang, J.; Ye, S.; Xing, W.; Yuan, X. Photocatalytic Degradation of Tetracycline Antibiotics Using Delafossite Silver Ferrite-Based Z-Scheme Photocatalyst: Pathways and Mechanism Insight. Chemosphere 2021, 270, 128651. [Google Scholar] [CrossRef]
- Shi, Z.; Zhang, Y.; Shen, X.; Duoerkun, G.; Zhu, B.; Zhang, L.; Li, M.; Chen, Z. Fabrication of G-C3N4/BiOBr Heterojunctions on Carbon Fibers as Weaveable Photocatalyst for Degrading Tetracycline Hydrochloride under Visible Light. Chem. Eng. J. 2020, 386, 124010. [Google Scholar] [CrossRef]
- Kundu, A.; Sharma, S.; Basu, S. Modulated BiOCl Nanoplates with Porous G-C3N4 Nanosheets for Photocatalytic Degradation of Color/Colorless Pollutants in Natural Sunlight. J. Phys. Chem. Solids 2021, 154, 110064. [Google Scholar] [CrossRef]
- Xiong, J.; Zeng, H.-Y.; Chen, C.-R.; Li, S.; Xu, S.; An, D.-S. Ag3VO4 Anchored ZnTi Hydrotalcite Microspheres with Rosette-like Structure for Tetracycline Degradation. Appl. Clay Sci. 2021, 208, 106118. [Google Scholar] [CrossRef]
- Sharma, S.; Basu, S. Highly Reusable Visible Light Active Hierarchical Porous WO3/SiO2 Monolith in Centimeter Length Scale for Enhanced Photocatalytic Degradation of Toxic Pollutants. Sep. Purif. Technol. 2020, 231, 115916. [Google Scholar] [CrossRef]
- Singla, S.; Shetti, N.P.; Basu, S.; Mondal, K.; Aminabhavi, T.M. Hydrogen Production Technologies—Membrane Based Separation, Storage and Challenges. J. Environ. Manag. 2022, 302, 113963. [Google Scholar] [CrossRef]
- Sharma, S.; Basu, S. Visible-Light-Driven Efficient Photocatalytic Abatement of Recalcitrant Pollutants by Centimeter-Length MoO3/SiO2 Monoliths with Long Service Life. Appl. Mater. Today 2021, 23, 101033. [Google Scholar] [CrossRef]
- Sadhasivam, S.; Anbarasan, N.; Gunasekaran, A.; Mukilan, M.; Jeganathan, K. Bi2S3 Entrenched BiVO4/WO3 Multidimensional Triadic Photoanode for Enhanced Photoelectrochemical Hydrogen Evolution Applications. Int. J. Hydrog. Energy 2022, 47, 14528–14541. [Google Scholar] [CrossRef]
- Liu, L.; Hu, T.; Dai, K.; Zhang, J.; Liang, C. A Novel Step-Scheme BiVO4/Ag3VO4 Photocatalyst for Enhanced Photocatalytic Degradation Activity under Visible Light Irradiation. Chin. J. Catal. 2020, 42, 46–55. [Google Scholar] [CrossRef]
- Chen, F.; Yang, Q.; Sun, J.; Yao, F.; Wang, S.; Wang, Y.; Wang, X.; Li, X.; Niu, C.; Wang, D.; et al. Enhanced Photocatalytic Degradation of Tetracycline by AgI/BiVO4 Heterojunction under Visible-Light Irradiation: Mineralization Efficiency and Mechanism. ACS Appl. Mater. Interfaces 2016, 48, 32887–32900. [Google Scholar] [CrossRef]
- Wang, Y.; Ding, K.; Xu, R.; Yu, D.; Wang, W.; Gao, P.; Liu, B. Fabrication of BiVO4/BiPO4/GO Composite Photocatalytic Material for the Visible Light-Driven Degradation. J. Clean. Prod. 2020, 247, 119108. [Google Scholar] [CrossRef]
- Samsudin, M.F.R.; Sufian, S. Hybrid 2D/3D g-C3N4/BiVO4 Photocatalyst Decorated with RGO for Boosted Photoelectrocatalytic Hydrogen Production from Natural Lake Water and Photocatalytic Degradation of Antibiotics. J. Mol. Liq. 2020, 314, 113530. [Google Scholar] [CrossRef]
- Samsudin, M.F.R.; Bacho, N.; Sufian, S.; Ng, Y.H. Photocatalytic Degradation of Phenol Wastewater over Z-Scheme g-C3N4/CNT/BiVO4 Heterostructure Photocatalyst under Solar Light Irradiation. J. Mol. Liq. 2019, 277, 977–988. [Google Scholar] [CrossRef]
- Zhang, L.; Meng, Y.; Dai, T.; Yao, Y.; Shen, H.; Xie, B.; Ni, Z.; Xia, S. Fabrication of a Coated BiVO4@LDHs Z-Scheme Heterojunction and Photocatalytic Degradation of Norfloxacin. Appl. Clay Sci. 2022, 219, 106435. [Google Scholar] [CrossRef]
- Lai, C.; Zhang, M.; Li, B.; Huang, D.; Zeng, G.; Qin, L.; Liu, X.; Yi, H.; Cheng, M.; Li, L.; et al. Fabrication of CuS/BiVO4 (0 4 0) Binary Heterojunction Photocatalysts with Enhanced Photocatalytic Activity for Ciprofloxacin Degradation and Mechanism Insight. Chem. Eng. J. 2019, 358, 891–902. [Google Scholar] [CrossRef]
- Sharma, S.; Basu, S. Fabrication of Centimeter-Sized Sb2S3/SiO2 Monolithic Mimosa Pudica Nanoflowers for Remediation of Hazardous Pollutants from Industrial Wastewater. J. Clean. Prod. 2021, 280, 124525. [Google Scholar] [CrossRef]
- Dashairya, L.; Sharma, S.; Rathi, A.; Saha, P.; Basu, S. Solar-Light-Driven Photocatalysis by Sb2S3/Carbon Based Composites towards Degradation of Noxious Organic Pollutants. Mater. Chem. Phys. 2021, 273, 125120. [Google Scholar] [CrossRef]
- Ayappan, C.; Jayaraman, V.; Palanivel, B.; Pandikumar, A.; Mani, A. Facile Preparation of Novel Sb2S3 Nanoparticles/Rod-like α-Ag2WO4 Heterojunction Photocatalysts: Continuous Modulation of Band Structure towards the Efficient Removal of Organic Contaminants. Sep. Purif. Technol. 2020, 236, 116302. [Google Scholar] [CrossRef]
- Cao, F.; Liu, W.; Zhou, L.; Deng, R.; Song, S.; Wang, S.; Su, S.; Zhang, H. Well-Defined Sb2S3 Microspheres: High-Yield Synthesis, Characterization, Their Optical and Electrochemical Hydrogen Storage Properties. Solid State Sci. 2011, 13, 1226–1231. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, Z.; Su, J. Sb2S3 Surface Modification for Improved Photoelectrochemical Water Splitting Performance of BiVO4 Photoanode. J. Photonics Energy 2021, 11, 016502. [Google Scholar] [CrossRef]
- Liu, C.; Zhou, J.; Su, J.; Guo, L. Turning the Unwanted Surface Bismuth Enrichment to Favourable BiVO4/BiOCl Heterojunction for Enhanced Photoelectrochemical Performance. Appl. Catal. B Environ. 2019, 241, 506–513. [Google Scholar] [CrossRef]
- Bai, Y.; Lu, J.; Bai, H.; Fang, Z.; Wang, F.; Liu, Y.; Sun, D.; Luo, B.; Fan, W.; Shi, W. Understanding the Key Role of Vanadium in P-Type BiVO4 for Photoelectrochemical N2 Fixation. Chem. Eng. J. 2021, 414, 128773. [Google Scholar] [CrossRef]
- Tian, Y.; An, Y.; Wei, H.; Wei, C.; Tao, Y.; Li, Y.; Xi, B.; Xiong, S.; Feng, J.; Qian, Y. Micron-Sized Nanoporous Vanadium Pentoxide Arrays for High-Performance Gel Zinc-Ion Batteries and Potassium Batteries. Chem. Mater. 2020, 32, 4054–4064. [Google Scholar] [CrossRef]
- Pang, Y.; Zhou, X.; Vovk, E.I.; Guan, C.; Li, S.; van Bavel, A.P.; Yang, Y. Understanding Lanthanum Oxide Surface Structure by DFT Simulation of Oxygen 1s Calibrated Binding Energy in XPS after in Situ Treatment. Appl. Surf. Sci. 2021, 548, 149214. [Google Scholar] [CrossRef]
- Luo, W.; Ao, X.; Li, Z.; Lv, L.; Li, J.; Hong, G.; Wu, Q.H.; Wang, C. Imbedding Ultrafine Sb2S3 Nanoparticles in Mesoporous Carbon Sphere for High-Performance Lithium-Ion Battery. Electrochim. Acta 2018, 290, 185–192. [Google Scholar] [CrossRef]
- Isari, A.A.; Mehregan, M.; Mehregan, S.; Hayati, F.; Rezaei Kalantary, R.; Kakavandi, B. Sono-Photocatalytic Degradation of Tetracycline and Pharmaceutical Wastewater Using WO3/CNT Heterojunction Nanocomposite under US and Visible Light Irradiations: A Novel Hybrid System. J. Hazard. Mater. 2020, 390, 122050. [Google Scholar] [CrossRef]
- Rajendran, S.; Khan, M.M.; Gracia, F.; Qin, J.; Gupta, V.K.; Arumainathan, S. Ce3+-Ion-Induced Visible-Light Photocatalytic Degradation and Electrochemical Activity of ZnO/CeO2 Nanocomposite. Sci. Rep. 2016, 6, 31641. [Google Scholar] [CrossRef] [Green Version]
- Zhao, W.; Feng, Y.; Huang, H.; Zhou, P.; Li, J.; Zhang, L.; Dai, B.; Xu, J.; Zhu, F.; Sheng, N.; et al. A Novel Z-Scheme Ag3VO4/BiVO4 Heterojunction Photocatalyst: Study on the Excellent Photocatalytic Performance and Photocatalytic Mechanism. Appl. Catal. B Environ. 2019, 245, 448–458. [Google Scholar] [CrossRef]
- Singla, S.; Basu, S.; Devi, P. Solar Light Responsive 2D/2D BiVO4/SnS2 Nanocomposite for Photocatalytic Elimination of Recalcitrant Antibiotics and Photoelectrocatalytic Water Splitting with High Performance. J. Ind. Eng. Chem. 2023, 118, 119–131. [Google Scholar] [CrossRef]
- Zhao, C.; Guo, J.; Yu, C.; Zhang, Z.; Sun, Z.; Piao, X. Fabrication of CNTs-Ag-TiO2 Ternary Structure for Enhancing Visible Light Photocatalytic Degradation of Organic Dye Pollutant. Mater. Chem. Phys. 2020, 248, 122873. [Google Scholar] [CrossRef]
- Zhang, R.; Cai, L.; Cai, Y.; Han, Q.; Li, Y.; Zhang, T.; Liu, Y.; Zeng, K.; Zhao, C.; Yu, J.; et al. Lamellar Insert SnS2 Anchored on BiOBr for Enhanced Photocatalytic Degradation of Organic Pollutant under Visible-Light. Colloids Surf. A Physicochem. Eng. Asp. 2021, 618, 126444. [Google Scholar] [CrossRef]
- Yang, L.; Wang, P.; Yin, J.; Wang, C.; Dong, G.; Wang, Y.; Ho, W. Engineering of Reduced Graphene Oxide on Nanosheet–g-C3N4/Perylene Imide Heterojunction for Enhanced Photocatalytic Redox Performance. Appl. Catal. B Environ. 2019, 250, 42–51. [Google Scholar] [CrossRef]
- Gnanasekaran, L.; Hemamalini, R.; Rajendran, S.; Qin, J.; Yola, M.L.; Atar, N.; Gracia, F. Nanosized Fe3O4 Incorporated on a TiO2 Surface for the Enhanced Photocatalytic Degradation of Organic Pollutants. J. Mol. Liq. 2019, 287, 11097. [Google Scholar] [CrossRef]
- Ali, W.; Ullah, H.; Zada, A.; Muhammad, W.; Ali, S.; Shaheen, S.; Alamgir, M.K.; Ansar, M.Z.; Khan, Z.U.; Bilal, H.; et al. Synthesis of TiO2 Modified Self-Assembled Honeycomb ZnO/SnO2 Nanocomposites for Exceptional Photocatalytic Degradation of 2,4-Dichlorophenol and Bisphenol A. Sci. Total Environ. 2020, 746, 141291. [Google Scholar] [CrossRef] [PubMed]
- Méndez-Romero, U.A.; Pérez-García, S.A.; Xu, X.; Wang, E.; Licea-Jiménez, L. Functionalized Reduced Graphene Oxide with Tunable Band Gap and Good Solubility in Organic Solvents. Carbon 2019, 146, 491–502. [Google Scholar] [CrossRef]
- Kamble, G.S.; Ling, Y.-C. Solvothermal Synthesis of Facet-Dependent BiVO4 Photocatalyst with Enhanced Visible-Light-Driven Photocatalytic Degradation of Organic Pollutant: Assessment of Toxicity by Zebrafish Embryo. Sci. Rep. 2020, 10, 12993. [Google Scholar] [CrossRef] [PubMed]
- Zamri, M.S.F.A.; Sapawe, N. Kinetic Study on Photocatalytic Degradation of Phenol Using Green Electrosynthesized TiO2 Nanoparticles. Mater. Today Proc. 2019, 19, 1261–1266. [Google Scholar] [CrossRef]
- Kamranifar, M.; Allahresani, A.; Naghizadeh, A. Synthesis and Characterizations of a Novel CoFe2O4@CuS Magnetic Nanocomposite and Investigation of Its Efficiency for Photocatalytic Degradation of Penicillin G Antibiotic in Simulated Wastewater. J. Hazard. Mater. 2019, 366, 545–555. [Google Scholar] [CrossRef]
- Monga, D.; Basu, S. Enhanced Photocatalytic Degradation of Industrial Dye by G-C3N4/TiO2 Nanocomposite: Role of Shape of TiO2. Adv. Powder Technol. 2019, 30, 1089–1098. [Google Scholar] [CrossRef]
- Wang, D.; Jia, F.; Wang, H.; Chen, F.; Fang, Y.; Dong, W.; Zeng, G.; Li, X.; Yang, Q.; Yuan, X. Simultaneously Efficient Adsorption and Photocatalytic Degradation of Tetracycline by Fe-Based MOFs. J. Colloid Interface Sci. 2018, 519, 273–284. [Google Scholar] [CrossRef]
- Nasseh, N.; Hossein Panahi, A.; Esmati, M.; Daglioglu, N.; Asadi, A.; Rajati, H.; Khodadoost, F. Enhanced Photocatalytic Degradation of Tetracycline from Aqueous Solution by a Novel Magnetically Separable FeNi3/SiO2/ZnO Nano-Composite under Simulated Sunlight: Efficiency, Stability, and Kinetic Studies. J. Mol. Liq. 2020, 301, 112434. [Google Scholar] [CrossRef]
- Wang, X.; Jia, J.; Wang, Y. Combination of Photocatalysis with Hydrodynamic Cavitation for Degradation of Tetracycline. Chem. Eng. J. 2017, 315, 272–284. [Google Scholar] [CrossRef]
- Gupta, D.; Chauhan, R.; Kumar, N.; Singh, V.; Srivastava, V.C.; Mohanty, P.; Mandal, T.K. Enhancing Photocatalytic Degradation of Quinoline by ZnO:TiO2 Mixed Oxide: Optimization of Operating Parameters and Mechanistic Study. J. Environ. Manag. 2020, 258, 110032. [Google Scholar] [CrossRef]
- Wang, X.; Jiang, L.; Li, K.; Wang, J.; Fang, D.; Zhang, Y.; Tian, D.; Zhang, Z.; Dionysiou, D.D. Fabrication of Novel Z-Scheme SrTiO3/MnFe2O4 System with Double-Response Activity for Simultaneous Microwave-Induced and Photocatalytic Degradation of Tetracycline and Mechanism Insight. Chem. Eng. J. 2020, 400, 125981. [Google Scholar] [CrossRef]
- Guo, P.; Zhao, F.; Hu, X. Boron- and Europium-Co-Doped g-C3N4 Nanosheets: Enhanced Photocatalytic Activity and Reaction Mechanism for Tetracycline Degradation. Ceram. Int. 2021, 47, 16256–16268. [Google Scholar] [CrossRef]
- Pan, Y.; Hu, X.; Bao, M.; Li, F.; Li, Y.; Lu, J. Fabrication of MIL-Fe (53)/Modified g-C3N4 Photocatalyst Synergy H2O2 for Degradation of Tetracycline. Sep. Purif. Technol. 2021, 279, 119661. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, X.; Sun, C.; Wan, J.; He, H.; Wang, F.; Dai, Y.; Yang, S.; Lin, Y.; Zhan, X. Insights into Removal of Tetracycline by Persulfate Activation with Peanut Shell Biochar Coupled with Amorphous Cu-Doped FeOOH Composite in Aqueous Solution. Environ. Sci. Pollut. Res. 2019, 26, 2820–2834. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, Y.; Xie, X.; Li, C.; Si, Y.; Zhang, M.; Yan, Q. Synthesis Flower-like BiVO4/BiOI Core/Shell Heterostructure Photocatalyst for Tetracycline Degradation under Visible-Light Irradiation. J. Mater. Sci. Mater. Electron. 2019, 30, 9311–9321. [Google Scholar] [CrossRef]
- Wang, Z.-H.; Jia, Y.-N.; Wu, X.-F.; Song, M.-C.; Ma, X.-Y.; Wang, H.; Zhang, X.-F. Preparation and Characterization of Bi2SiO5/BiVO4 n–n Isotype Heterojunction Composites as a Visible-Light-Induced Photocatalyst for Tetracycline and Levofloxacin Degradation. J. Mater. Sci. Mater. Electron. 2023, 34, 433. [Google Scholar] [CrossRef]
- Xiao, Y.; Wang, H.; Jiang, Y.; Zhang, W.; Zhang, J.; Wu, X.; Liu, Z.; Deng, W. Hierarchical Sb2S3/ZnIn2S4 Core–Shell Heterostructure for Highly Efficient Photocatalytic Hydrogen Production and Pollutant Degradation. J. Colloid Interface Sci. 2022, 623, 109–123. [Google Scholar] [CrossRef]
- Zhang, J.; Deng, M.; Ren, F.; Wu, Y.; Wang, Y. Effects of Mo/W Codoping on the Visible-Light Photocatalytic Activity of Monoclinic BiVO4 within the GGA + U Framework. RSC Adv. 2016, 6, 12290–12297. [Google Scholar] [CrossRef]
- Yang, H.; Li, M.; Fu, L.; Tang, A.; Mann, S. Controlled Assembly of Sb2S3 Nanoparticles on Silica/Polymer Nanotubes: Insights into the Nature of Hybrid Interfaces. Sci. Rep. 2013, 3, 1336. [Google Scholar] [CrossRef] [Green Version]









| Sample | Specific Surface Area (m2/g) | Mean Pore Volume (cm3/g) | Mean Pore Diameter (nm) |
|---|---|---|---|
| BV | 33 | 0.582 | 13.81 |
| SBS | 12 | 0.187 | 9.72 |
| 11BVSBS | 38 | 0.242 | 11.10 |
| 13BVSBS | 42 | 0.481 | 14.52 |
| 31BVSBS | 39 | 0.372 | 11.70 |
| 15BVSBS | 43 | 0.521 | 14.96 |
| Photocatalyst | Degradation (%) | Rate Constant (min−1) | Synergy (R) Factor |
|---|---|---|---|
| TiO2-P25 | 47.0 | 0.00413 ± 2.57 × 10−4 | - |
| BV | 49.1 | 0.00419 ± 5.84 × 10−5 | - |
| SBS | 51.2 | 0.00447 ± 1.54 × 10−4 | - |
| 11BVSBS | 58.9 | 0.00559 ± 1.62 × 10−4 | 0.64 |
| 13BVSBS | 88.7 | 0.01557 ± 9.57 × 10−4 | 1.75 |
| 31BVSBS | 66.5 | 0.00674 ± 2.60 × 10−4 | 0.77 |
| 15BVSBS | 89.6 | 0.01655 ± 9.15 × 10−4 | 1.91 |
| No catalyst | 8.90 | 0.00079 ± 1.46 × 10−4 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singla, S.; Devi, P.; Basu, S. Highly Effectual Photocatalytic Remediation of Tetracycline under the Broad Spectrum of Sunlight by Novel BiVO4/Sb2S3 Nanocomposite. Catalysts 2023, 13, 731. https://doi.org/10.3390/catal13040731
Singla S, Devi P, Basu S. Highly Effectual Photocatalytic Remediation of Tetracycline under the Broad Spectrum of Sunlight by Novel BiVO4/Sb2S3 Nanocomposite. Catalysts. 2023; 13(4):731. https://doi.org/10.3390/catal13040731
Chicago/Turabian StyleSingla, Shelly, Pooja Devi, and Soumen Basu. 2023. "Highly Effectual Photocatalytic Remediation of Tetracycline under the Broad Spectrum of Sunlight by Novel BiVO4/Sb2S3 Nanocomposite" Catalysts 13, no. 4: 731. https://doi.org/10.3390/catal13040731