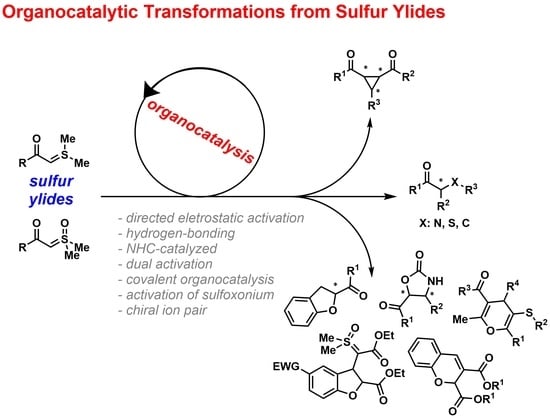
Organocatalytic Transformations from Sulfur Ylides
Abstract
:1. Introduction
2. Organocatalytic Corey–Chaykovsky Reaction
3. Organocatalytic Formal C–H, N–H, and S–H Insertion
4. Cyclization
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 18-crown-6 | 1,4,7,10,13,16-hexaoxacyclooctadecane |
| AcO | acetyl |
| Å | Angstrom |
| aq | aqueous |
| Ar | aryl |
| Bn | benzyl |
| Bu | butyl |
| cat | catalyst |
| DABCO | 1,4-diazabicyclo[2.2.2]octane |
| Dec | decanyl |
| DMAP | N,N-4-dimethylaminopyridine |
| DMSO | dimethylsulfoxide |
| de | diastereomeric excess |
| dr | diastereomeric ratio |
| EDG | electron directing group |
| ee | enantiomeric excess |
| eq | equivalent |
| er | enatiomeric ratio |
| Et | ethyl |
| EWG | electron withdrawing group |
| i | iso |
| m | mili |
| M | molar (mol·L−1) |
| Me | methyl |
| MS | molecular sieves |
| n | normal |
| NHC | N-heterocyclic carbene |
| o | ortho |
| Ph | phenyl |
| Pr | propyl |
| QM | quinone methide |
| rt | room temperature |
| t | tert |
| TBS | tert-butyldimethylsilyl |
| TMS | trimethylsilyl |
| y | yield |
References and Notes
- The Nobel Prize in Chemistry 2021. Available online: https://www.nobelprize.org/prizes/chemistry/2021/summary/ (accessed on 8 December 2022).
- Dondoni, A.; Massi, A. Asymmetric Organocatalysis: From Infancy to Adolescence. Angew. Chem. Int. Ed. 2008, 47, 4638–4660. [Google Scholar] [CrossRef] [PubMed]
- Dalko, P.I.; Moisan, L. In the Golden Age of Organocatalysis. Angew. Chem. Int. Ed. 2004, 43, 5138–5175. [Google Scholar] [CrossRef]
- Marqués-López, E.; Herrera, R.P.; Christmann, M. Asymmetric Organocatalysis in Total Synthesis—A Trial by Fire. Nat. Prod. Rep. 2010, 27, 1138–1167. [Google Scholar] [CrossRef] [PubMed]
- Grondal, C.; Jeanty, M.; Enders, D. Organocatalytic Cascade Reactions as a New Tool in Total Synthesis. Nat. Chem. 2010, 2, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; He, X.-H.; Liu, Y.-Q.; He, G.; Peng, C.; Li, J.-L. Asymmetric Organocatalysis: An Enabling Technology for Medicinal Chemistry. Chem. Soc. Rev. 2021, 50, 1522–1586. [Google Scholar] [CrossRef]
- García Mancheño, O.; Waser, M. Recent Developments and Trends in Asymmetric Organocatalysis. Eur. J. Org. Chem. 2022, 26, e202200950. [Google Scholar] [CrossRef]
- Dalko, P.I. Enantioselective Organocatalysis: Reactions and Experimental Procedures; Wiley-VCH; John Wiley: Weinheim, Germany; Chichester, UK, 2007. [Google Scholar]
- Ingold, C.K.; Jessop, J.A. XCV.—Influence of Poles and Polar Linkings on the Course Pursued by Elimination Reactions. Part IX. Isolation of a Substance Believed to Contain a Semipolar Double Linking with Participating Carbon. J. Chem. Soc. 1930, 713–718. [Google Scholar] [CrossRef]
- Johnson, A.W.; LaCount, R.B. The Chemistry of Ylids. VI. Dimethylsulfonium Fluorenylide—A Synthesis of Epoxides. J. Am. Chem. Soc. 1961, 83, 417–423. [Google Scholar] [CrossRef]
- Corey, E.J.; Chaykovsky, M. Dimethylsulfoxonium Methylide. J. Am. Chem. Soc. 1962, 84, 867–868. [Google Scholar] [CrossRef]
- Corey, E.J.; Chaykovsky, M. Dimethylsulfonium Methylide, a Reagent for Selective Oxirane Synthesis from Aldehydes and Ketones. J. Am. Chem. Soc. 1962, 84, 3782–3783. [Google Scholar] [CrossRef]
- Corey, E.J.; Chaykovsky, M. Dimethyloxosulfonium Methylide ((CH3)2SOCH2) and Dimethylsulfonium Methylide ((CH3)2SCH2). Formation and Application to Organic Synthesis. J. Am. Chem. Soc. 1965, 87, 1353–1364. [Google Scholar] [CrossRef]
- Lu, L.-Q.; Li, T.-R.; Wang, Q.; Xiao, W.-J. Beyond Sulfide-Centric Catalysis: Recent Advances in the Catalytic Cyclization Reactions of Sulfur Ylides. Chem. Soc. Rev. 2017, 46, 4135–4149. [Google Scholar] [CrossRef] [PubMed]
- Burtoloso, A.C.B.; Dias, R.M.P.; Leonarczyk, I.A. Sulfoxonium and Sulfonium Ylides as Diazocarbonyl Equivalents in Metal-Catalyzed Insertion Reactions: Sulfoxonium and Sulfonium Ylides in Insertion Reactions. Eur. J. Org. Chem. 2013, 2013, 5005–5016. [Google Scholar] [CrossRef]
- Belkin, Y.V.; Polezhaeva, N.A. The Chemistry of Stabilised Sulphonium Ylides. Russ. Chem. Rev. 1981, 50, 481–497. [Google Scholar] [CrossRef]
- Bisag, G.D.; Ruggieri, S.; Fochi, M.; Bernardi, L. Sulfoxonium Ylides: Simple Compounds with Chameleonic Reactivity. Org. Biomol. Chem. 2020, 18, 8793–8809. [Google Scholar] [CrossRef] [PubMed]
- Caiuby, C.A.D.; Furniel, L.G.; Burtoloso, A.C.B. Asymmetric Transformations from Sulfoxonium Ylides. Chem. Sci. 2022, 13, 1192–1209. [Google Scholar] [CrossRef]
- Neuhaus, J.D.; Oost, R.; Merad, J.; Lady, N. Sulfur-Based Ylides in Transition-Metal-Catalysed Processes. Top. Curr. Chem. 2018, 376, 15. [Google Scholar] [CrossRef] [Green Version]
- Li, A.-H.; Dai, L.-X.; Aggarwal, V.K. Asymmetric Ylide Reactions: Epoxidation, Cyclopropanation, Aziridination, Olefination, and Rearrangement. Chem. Rev. 1997, 97, 2341–2372. [Google Scholar] [CrossRef]
- Aggarwal, V.K.; Winn, C.L. Catalytic, Asymmetric Sulfur Ylide-Mediated Epoxidation of Carbonyl Compounds: Scope, Selectivity, and Applications in Synthesis. Acc. Chem. Res. 2004, 37, 611–620. [Google Scholar] [CrossRef]
- McGarrigle, E.M.; Myers, E.L.; Illa, O.; Shaw, M.A.; Riches, S.L.; Aggarwal, V.K. Chalcogenides as Organocatalysts. Chem. Rev. 2007, 107, 5841–5883. [Google Scholar] [CrossRef]
- Fan, Y.-Y.; Gao, X.-H.; Yue, J.-M. Attractive Natural Products with Strained Cyclopropane and/or Cyclobutane Ring Systems. Sci. China Chem. 2016, 59, 1126–1141. [Google Scholar] [CrossRef]
- Ebner, C.; Carreira, E.M. Cyclopropanation Strategies in Recent Total Syntheses. Chem. Rev. 2017, 117, 11651–11679. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Lin, Z.; Jiang, H. Recent Advances in the Synthesis of Cyclopropanes. Org. Biomol. Chem. 2018, 16, 7315–7329. [Google Scholar] [CrossRef] [PubMed]
- Charette, A.B.; Beauchemin, A. Simmons-Smith Cyclopropanation Reaction. In Organic Reactions; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2001; pp. 1–415. [Google Scholar]
- Lebel, H.; Marcoux, J.-F.; Molinaro, C.; Charette, A.B. Stereoselective Cyclopropanation Reactions. Chem. Rev. 2003, 103, 977–1050. [Google Scholar] [CrossRef] [PubMed]
- Kunz, R.K.; MacMillan, D.W.C. Enantioselective Organocatalytic Cyclopropanations. The Identification of a New Class of Iminium Catalyst Based upon Directed Electrostatic Activation. J. Am. Chem. Soc. 2005, 127, 3240–3241. [Google Scholar] [CrossRef] [Green Version]
- Hartikka, A.; Arvidsson, P.I. Tetrazolic Acid Functionalized Dihydroindol: Rational Design of a Highly Selective Cyclopropanation Organocatalyst. J. Org. Chem. 2007, 72, 5874–5877. [Google Scholar] [CrossRef]
- Hartikka, A.; Ślósarczyk, A.T.; Arvidsson, P.I. Application of Novel Sulfonamides in Enantioselective Organocatalyzed Cyclopropanation. Tetrahedron Asymmetry 2007, 18, 1403–1409. [Google Scholar] [CrossRef]
- Cheng, Y.; An, J.; Lu, L.-Q.; Luo, L.; Wang, Z.-Y.; Chen, J.-R.; Xiao, W.-J. Asymmetric Cyclopropanation of β,γ-Unsaturated α-Ketoesters with Stabilized Sulfur Ylides Catalyzed by C2-Symmetric Ureas. J. Org. Chem. 2011, 76, 281–284. [Google Scholar] [CrossRef]
- Taylor, M.S.; Jacobsen, E.N. Asymmetric Catalysis by Chiral Hydrogen-Bond Donors. Angew. Chem. Int. Ed. 2006, 45, 1520–1543. [Google Scholar] [CrossRef]
- Biswas, A.; De Sarkar, S.; Tebben, L.; Studer, A. Enantioselective Cyclopropanation of Enals by Oxidative N-Heterocyclic Carbene Catalysis. Chem. Commun. 2012, 48, 5190–5192. [Google Scholar] [CrossRef]
- Flanigan, D.M.; Romanov-Michailidis, F.; White, N.A.; Rovis, T. Organocatalytic Reactions Enabled by N-Heterocyclic Carbenes. Chem. Rev. 2015, 115, 9307–9387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Risi, C.; Brandolese, A.; Di Carmine, G.; Ragno, D.; Massi, A.; Bortolini, O. Oxidative N-Heterocyclic Carbene Catalysis. Chem. Eur. J. 2023, 29, e202202467. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, X.; Dong, S.; Lin, L.; Feng, X. Asymmetric Organocatalytic Cyclopropanation of Cinnamone Derivatives with Stabilized Sulfonium Ylides. J. Org. Chem. 2013, 78, 6322–6327. [Google Scholar] [CrossRef]
- Bisag, G.D.; Pecchini, P.; Mancinelli, M.; Fochi, M.; Bernardi, L. Sulfoxonium Ylides in Aminocatalysis: An Enantioselective Entry to Cyclopropane-Fused Chromanol Structures. Org. Lett. 2022, 24, 5468–5473. [Google Scholar] [CrossRef] [PubMed]
- Yudin, A.K. (Ed.) Aziridines and Epoxides in Organic Synthesis, 1st ed.; Wiley: Hoboken, NJ, USA, 2006. [Google Scholar]
- Kavanagh, S.A.; Piccinini, A.; Fleming, E.M.; Connon, S.J. Urea Derivatives Are Highly Active Catalysts for the Base-Mediated Generation of Terminal Epoxides from Aldehydes and Trimethylsulfonium Iodide. Org. Biomol. Chem. 2008, 6, 1339–1343. [Google Scholar] [CrossRef] [Green Version]
- Baldwin, J.E.; Adlington, R.M.; Godfrey, C.R.A.; Gollins, D.W.; Smith, M.L.; Russel, A.T. A New Approach to the Synthesis of γ-Keto-α-Amino Acids: Synthesis of Optically Pure 5-Hydroxy-4-Oxo-L-Norvaline, L-HON. Synlett 1993, 1993, 51–53. [Google Scholar] [CrossRef]
- Baldwin, J.E.; Adlington, R.M.; Godfrey, C.R.A.; Gollins, D.W.; Vaughan, J.G. A Novel Entry to Carbenoid Species via β-Ketosulfoxonium Ylides. J. Chem. Soc. Chem. Commun. 1993, 18, 1434–1435. [Google Scholar] [CrossRef]
- Mangion, I.K.; Ruck, R.T.; Rivera, N.; Huffman, M.A.; Shevlin, M. A Concise Synthesis of a β-Lactamase Inhibitor. Org. Lett. 2011, 13, 5480–5483. [Google Scholar] [CrossRef]
- Molinaro, C.; Bulger, P.G.; Lee, E.E.; Kosjek, B.; Lau, S.; Gauvreau, D.; Howard, M.E.; Wallace, D.J.; O’Shea, P.D. CRTH2 Antagonist MK-7246: A Synthetic Evolution from Discovery through Development. J. Org. Chem. 2012, 77, 2299–2309. [Google Scholar] [CrossRef]
- Ruck, R.T.; Pan, J.; Vaswani, R.G.; Kosjek, B.; Strotman, N.A.; Cai, C.; Humphrey, G.R. Harnessing the Power of Catalysis for the Synthesis of CRTH2 Antagonist MK-1029. Org. Process Res. Dev. 2022, 26, 648–656. [Google Scholar] [CrossRef]
- Talero, A.G.; Martins, B.S.; Burtoloso, A.C.B. Coupling of Sulfoxonium Ylides with Arynes: A Direct Synthesis of Pro-Chiral Aryl Ketosulfoxonium Ylides and Its Application in the Preparation of α-Aryl Ketones. Org. Lett. 2018, 20, 7206–7211. [Google Scholar] [CrossRef] [PubMed]
- Janot, C.; Palamini, P.; Dobson, B.C.; Muir, J.; Aïssa, C. Palladium-Catalyzed Synthesis of Bis-Substituted Sulfoxonium Ylides. Org. Lett. 2019, 21, 296–299. [Google Scholar] [CrossRef] [PubMed]
- Janot, C.; Chagnoleau, J.-B.; Halcovitch, N.R.; Muir, J.; Aïssa, C. Palladium-Catalyzed Synthesis of α-Carbonyl-A′-(Hetero)Aryl Sulfoxonium Ylides: Scope and Insight into the Mechanism. J. Org. Chem. 2020, 85, 1126–1137. [Google Scholar] [CrossRef] [PubMed]
- Momo, P.B.; Leveille, A.N.; Farrar, E.H.E.; Grayson, M.N.; Mattson, A.E.; Burtoloso, A.C.B. Enantioselective S–H Insertion Reactions of A-Carbonyl Sulfoxonium Ylides. Angew. Chem. Int. Ed. 2020, 59, 15554–15559. [Google Scholar] [CrossRef]
- Fang, X.; Wang, C.-J. Recent Advances in Asymmetric Organocatalysis Mediated by Bifunctional Amine–Thioureas Bearing Multiple Hydrogen-Bonding Donors. Chem. Commun. 2015, 51, 1185–1197. [Google Scholar] [CrossRef]
- Leveille, A.N.; Echemendía, R.; Mattson, A.E.; Burtoloso, A.C.B. Enantioselective Indole Insertion Reactions of α-Carbonyl Sulfoxonium Ylides. Org. Lett. 2021, 23, 9446–9450. [Google Scholar] [CrossRef]
- Guo, W.; Luo, Y.; Sung, H.H.-Y.; Williams, I.D.; Li, P.; Sun, J. Chiral Phosphoric Acid Catalyzed Enantioselective Synthesis of α-Tertiary Amino Ketones from Sulfonium Ylides. J. Am. Chem. Soc. 2020, 142, 14384–14390. [Google Scholar] [CrossRef]
- Furniel, L.G.; Burtoloso, A.C.B. Copper-Catalyzed N–H Insertion Reactions from Sulfoxonium Ylides. Tetrahedron 2020, 76, 131313. [Google Scholar] [CrossRef]
- Furniel, L.G.; Echemendía, R.; Burtoloso, A.C.B. Cooperative Copper-Squaramide Catalysis for the Enantioselective N–H Insertion Reaction with Sulfoxonium Ylides. Chem. Sci. 2021, 12, 7453–7459. [Google Scholar] [CrossRef]
- Guo, W.; Wang, M.; Han, Z.; Huang, H.; Sun, J. Organocatalytic Asymmetric Synthesis of α-Amino Esters from Sulfoxonium Ylides. Chem. Sci. 2021, 12, 11191–11196. [Google Scholar] [CrossRef]
- Guo, W.; Jiang, F.; Li, S.; Sun, J. Organocatalytic Asymmetric Azidation of Sulfoxonium Ylides: Mild Synthesis of Enantioenriched α-Azido Ketones Bearing a Labile Tertiary Stereocenter. Chem. Sci. 2022, 13, 11648–11655. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.-Q.; Cao, Y.-J.; Liu, X.-P.; An, J.; Yao, C.-J.; Ming, Z.-H.; Xiao, W.-J. A New Entry to Cascade Organocatalysis: Reactions of Stable Sulfur Ylides and Nitroolefins Sequentially Catalyzed by Thiourea and DMAP. J. Am. Chem. Soc. 2008, 130, 6946–6948. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.-Q.; Li, F.; An, J.; Zhang, J.-J.; An, X.-L.; Hua, Q.-L.; Xiao, W.-J. Construction of Fused Heterocyclic Architectures by Formal [4 + 1]/[3 + 2] Cycloaddition Cascade of Sulfur Ylides and Nitroolefins. Angew. Chem. Int. Ed. 2009, 48, 9542–9545. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.-Q.; Li, F.; An, J.; Cheng, Y.; Chen, J.-R.; Xiao, W.-J. Hydrogen-Bond-Mediated Asymmetric Cascade Reaction of Stable Sulfur Ylides with Nitroolefins: Scope, Application and Mechanism. Chem. Eur. J. 2012, 18, 4073–4079. [Google Scholar] [CrossRef]
- Yang, Q.-Q.; Xiao, W.-J. Catalytic Asymmetric Synthesis of Chiral Dihydrobenzofurans through a Formal [4 + 1] Annulation Reaction of Sulfur Ylides and In Situ Generated Ortho-Quinone Methides. Eur. J. Org. Chem. 2017, 2017, 233–236. [Google Scholar] [CrossRef]
- Li, K.; Hu, J.; Liu, H.; Tong, X. Amine-Catalyzed Formal (3 + 3) Annulations of 2-(Acetoxymethyl)Buta-2,3-Dienoate with Sulfur Ylides: Synthesis of 4H-Pyrans Bearing a Vinyl Sulfide Group. Chem. Commun. 2012, 48, 2900. [Google Scholar] [CrossRef]
- Denisa Bisag, G.; Ruggieri, S.; Fochi, M.; Bernardi, L. Catalyst- and Substrate-Dependent Chemodivergent Reactivity of Stabilised Sulfur Ylides with Salicylaldehydes. Adv. Synth. Catal. 2021, 363, 3053–3059. [Google Scholar] [CrossRef]
- Day, D.P.; Vargas, J.A.M.; Burtoloso, A.C.B. Synthetic Routes Towards the Synthesis of Geminal A-Difunctionalized Ketones. Chem. Rec. 2021, 21, 2837–2854. [Google Scholar] [CrossRef]
- After we have submitted our manuscript, a review about applications of sulfonium and sulfoxonium ylides in enantioselective organocatalyzed reactions was also published in the literature. Wang, Z.-H.; Sun, T.-J.; Zhang, Y.-P.; You, Y.; Zhao, J.-Q.; Yin, J.-Q.; Yuan, W.-C. Application of Sulfonium and Sulfoxonium Ylides in Organocatalyzed Asymmetric Reaction. Chem. Synth. 2023, 3, 12. [Google Scholar] [CrossRef]




































Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hayashi, M.; Burtoloso, A.C.B. Organocatalytic Transformations from Sulfur Ylides. Catalysts 2023, 13, 689. https://doi.org/10.3390/catal13040689
Hayashi M, Burtoloso ACB. Organocatalytic Transformations from Sulfur Ylides. Catalysts. 2023; 13(4):689. https://doi.org/10.3390/catal13040689
Chicago/Turabian StyleHayashi, Marcio, and Antonio C. B. Burtoloso. 2023. "Organocatalytic Transformations from Sulfur Ylides" Catalysts 13, no. 4: 689. https://doi.org/10.3390/catal13040689