Solvent-Free Aldol Condensation of Cyclopentanone with Natural Clay-Based Catalysts: Origin of Activity & Selectivity
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of the Synthesized SO3H-APG
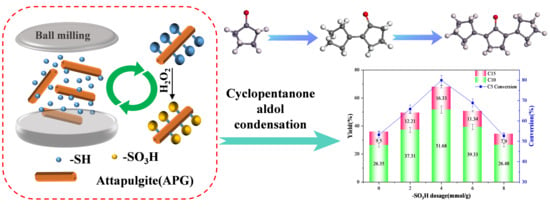
2.2. Screening of Reaction Conditions
2.3. Response Surface Methodology (RSM) Analysis
2.4. Reaction Kinetic Analysis
2.5. The Mechanism of Acid–Base Bifunctional Catalyst Action
2.6. Catalyst Stability and Recycling
3. Materials and Method
3.1. Materials and Equipment
3.2. Preparation of SO3H-APG Catalyst
3.3. Catalyst Characterization
3.4. Aldol Condensation of Cyclopentanone
3.5. Products Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Choe, B.; Lee, S.; Won, W. Process integration and optimization for economical production of commodity chemicals from lignocellulosic biomass. Renew. Energy 2020, 162, 242–248. [Google Scholar] [CrossRef]
- Huchede, M.; Lorentz, C.; Cardenas, L.; Morvan, D.; Belliere-Baca, V.; Millet, J.M.M. Gas phase dehydration of 3-hydroxybutanone on orthophosphate catalysts for bio-based production of butenone for a sustainable industrial route to vitamin A. J. Ind. Eng. Chem. 2020, 88, 178–185. [Google Scholar] [CrossRef]
- Jiang, Y.; Liu, H.; Zhao, H.; Zhou, R.; Du, W.; Wang, S.; Hou, Z. Synthesis of 3-hydroxybutyraldehyde over highly stable solid base catalysts prepared from layered double hydroxides. Appl. Clay Sci. 2021, 214, 106277. [Google Scholar] [CrossRef]
- Niu, Y.; Lv, Y.; Lei, Y.; Liu, S.; Liang, Y.; Wang, D.; Hui, S.e. Biomass torrefaction: Properties, applications, challenges, and economy. Renew. Sustain. Energy Rev. 2019, 115, 109395. [Google Scholar] [CrossRef]
- Phromphithak, S.; Meepowpan, P.; Shimpalee, S.; Tippayawong, N. Transesterification of palm oil into biodiesel using ChOH ionic liquid in a microwave heated continuous flow reactor. Renew. Energy 2020, 154, 925–936. [Google Scholar] [CrossRef]
- Tempelman, C.H.L.; Jacobs, J.F.; Ramkhelawan, S.; Mok, A.; van der Zalm, W.; Degirmenci, V. Processing of agricultural apple fruit waste into sugar rich feedstocks for the catalytic production of 5-HMF over a Sn Amberlyst-15 resin catalyst. J. Ind. Eng. Chem. 2021, 99, 443–448. [Google Scholar] [CrossRef]
- Hita, I.; Cordero-Lanzac, T.; Bonura, G.; Cannilla, C.; Arandes, J.M.; Frusteri, F.; Bilbao, J. Hydrodeoxygenation of raw bio-oil towards platform chemicals over FeMoP/zeolite catalysts. J. Ind. Eng. Chem. 2019, 80, 392–400. [Google Scholar] [CrossRef] [Green Version]
- Jing, Y.; Xin, Y.; Guo, Y.; Liu, X.; Wang, Y. Highly efficient Nb2O5 catalyst for aldol condensation of biomass-derived carbonyl molecules to fuel precursors. Chin. J. Catal. 2019, 40, 1168–1177. [Google Scholar] [CrossRef]
- Shao, S.; Dong, W.; Li, X.; Zhang, H.; Xiao, R.; Cai, Y. Solvent-free synthesis of jet fuel by aldol condensation and hydroprocessing of cyclopentanone as biomass-derivates. J. Clean. Prod. 2020, 250, 119459. [Google Scholar] [CrossRef]
- Sheng, X.; Xu, Q.; Wang, X.; Li, N.; Jia, H.; Shi, H.; Niu, M.; Zhang, J.; Ping, Q. Waste Seashells as a Highly Active Catalyst for Cyclopentanone Self-Aldol Condensation. Catalysts 2019, 9, 661. [Google Scholar] [CrossRef] [Green Version]
- Yan, L.; Zhang, J.; Gao, X.; Song, F.; Wang, X.; Zhang, T.; Liu, X.; Meng, X.; Zhang, Q.; Han, Y.; et al. Oxidative coupling of methane over Mo-Sn catalysts. Chem. Commun. 2021, 57, 13297–13300. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Zhang, J.-L.; Hu, H.-M.; Wang, F.; Bai, C.; Li, X.-Y.; Wang, X.; Wang, B.-Z. Structural diversity and near-infrared luminescence of lanthanide coordination polymers with different flexibility and coordination orientation based on bipyridyl carboxylate and dicarboxylate ligands. J. Solid State Chem. 2020, 292, 121654. [Google Scholar] [CrossRef]
- Kunkes, E.L.; Simonetti, D.A.; West, R.M.; Serrano-Ruiz, J.C.; Gartner, C.A.; Dumesic, J.A. Catalytic conversion of biomass to monofunctional hydrocarbons and targeted liquid-fuel classes. Science 2008, 322, 417–421. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Lei, H.; Zhu, L.; Wu, J.; Chen, S. From lignocellulosic biomass to renewable cycloalkanes for jet fuels. Green Chem. 2015, 17, 4736–4747. [Google Scholar] [CrossRef]
- Bano, K.; Jain, A.; Sarkar, R.; Panda, T.K. Economically Viable and Efficient Catalysts for Esterification and Cross Aldol Condensation Reactions under Mild Conditions. Chemistryselect 2020, 5, 4470–4477. [Google Scholar] [CrossRef]
- Deng, Q.; Nie, G.; Pan, L.; Zou, J.-J.; Zhang, X.; Wang, L. Highly selective self-condensation of cyclic ketones using MOF-encapsulating phosphotungstic acid for renewable high-density fuel. Green Chem. 2015, 17, 4473–4481. [Google Scholar] [CrossRef]
- Han, P.; Nie, G.; Xie, J.; E, X.-T.-F.; Pan, L.; Zhang, X.; Zou, J.-J. Synthesis of high-density biofuel with excellent low-temperature properties from lignocellulose-derived feedstock. Fuel Process. Technol. 2017, 163, 45–50. [Google Scholar] [CrossRef]
- Wang, H.; Wang, Z.; Wang, S.; Yang, C.; Li, S.; Gao, P.; Sun, Y. The effect of the particle size on Fischer-Tropsch synthesis for ZSM-5 zeolite supported cobalt-based catalysts. Chem. Commun. 2021, 57, 13522–13525. [Google Scholar] [CrossRef]
- Cui, X.; Zhao, X.; Liu, D. A novel route for the flexible preparation of hydrocarbon jet fuels from biomass-based platform chemicals: A case of using furfural and 2,3-butanediol as feedstocks. Green Chem. 2018, 20, 2018–2026. [Google Scholar] [CrossRef]
- Veloso, C.O.; Perez, C.N.; de Souza, B.M.; Lima, E.C.; Dias, A.G.; Monteiro, J.L.F.; Henriques, C.A. Condensation of glyceraldehyde over Mg,Al-mixed oxides derived from hydrotalcites. Microporous Mesoporous Mater. 2008, 107, 23–30. [Google Scholar] [CrossRef]
- Collier, V.E.; Ellebracht, N.C.; Lindy, G.I.; Moschetta, E.G.; Jones, C.W. Kinetic and Mechanistic Examination of Acid-Base Bifunctional Aminosilica Catalysts in Aldol and Nitroaldol Condensations. ACS Catal. 2016, 6, 460–468. [Google Scholar] [CrossRef]
- Faba, L.; Diaz, E.; Ordonez, S. Hydrodeoxygenation of acetone-furfural condensation adducts over alumina-supported noble metal catalysts. Appl. Catal. B-Environ. 2014, 160, 436–444. [Google Scholar] [CrossRef] [Green Version]
- Hronec, M.; Fulajtarova, K.; Liptaj, T.; Stolcova, M.; Pronayova, N.; Sotak, T. Cyclopentanone: A raw material for production of C-15 and C-17 fuel precursors. Biomass Bioenergy 2014, 63, 291–299. [Google Scholar] [CrossRef]
- Cueto, J.; Faba, L.; Diaz, E.; Ordonez, S. Cyclopentanone as an Alternative Linking Reactant for Heterogeneously Catalyzed Furfural Aldol Condensation. Chemcatchem 2017, 9, 1765–1770. [Google Scholar] [CrossRef] [Green Version]
- Huang, R.; Chang, J.; Choi, H.; Vohs, J.M.; Gorte, R.J. Furfural Upgrading by Aldol Condensation with Ketones over Solid-Base Catalysts. Catal. Lett. 2022, 152, 3833–3842. [Google Scholar] [CrossRef]
- de Reviere, A.; Gunst, D.; Sabbe, M.; Verberckmoes, A. Sustainable short-chain olefin production through simultaneous dehydration of mixtures of 1-butanol and ethanol over HZSM-5 and gamma-Al2O3. J. Ind. Eng. Chem. 2020, 89, 257–272. [Google Scholar] [CrossRef]
- Diaz-Sanchez, M.; Gomez, I.J.; Prashar, S.; Horacek, M.; Lamac, M.; Urban, B.; Pinkas, J.; Gomez-Ruiz, S. Multifunctional catalysts based on palladium nanoparticles supported on functionalized halloysites: Applications in catalytic C-C coupling, selective oxidation and dehalogenation reactions. Appl. Clay Sci. 2021, 214, 106272. [Google Scholar] [CrossRef]
- Sudarsanam, P.; Peeters, E.; Makshina, E.V.; Parvulescu, V.I.; Sels, B.F. Advances in porous and nanoscale catalysts for viable biomass conversion. Chem. Soc. Rev. 2019, 48, 2366–2421. [Google Scholar] [CrossRef] [PubMed]
- Vaculikova, L.; Valovicova, V.; Plevova, A.E.; Napruszewska, B.D.; Duraczynska, D.; Karcz, R.; Serwicka, E.M. Synthesis, characterization and catalytic activity of cryptomelane/montmorillonite composites. Appl. Clay Sci. 2021, 202, 105977. [Google Scholar] [CrossRef]
- Shao, S.; Hu, X.; Dong, W.; Li, X.; Zhang, H.; Xiao, R.; Cai, Y. Integrated C-C coupling/hydrogenation of ketones derived from biomass pyrolysis for aviation fuel over Ni/Mg-Al-O/AC bifunctional catalysts. J. Clean. Prod. 2021, 282, 124331. [Google Scholar] [CrossRef]
- Zeidan, R.K.; Davis, M.E. The effect of acid-base pairing on catalysis: An efficient acid-base functionalized catalyst for aldol condensation. J. Catal. 2007, 247, 379–382. [Google Scholar] [CrossRef]
- Zhao, X.; Li, S.; Hu, Y.; Zhang, X.; Chen, L.; Wang, C.; Ma, L.; Zhang, Q. Synthesis of long chain alkanes via aldol condensation over modified chitosan catalyst and subsequent hydrodeoxygenation. Chem. Eng. J. 2022, 428, 131368. [Google Scholar] [CrossRef]
- Amarasekara, A.S.; Wiredu, B.; Grady, T.L.; Obregon, R.G.; Margetic, D. Solid acid catalyzed aldol dimerization of levulinic acid for the preparation of C10 renewable fuel and chemical feedstocks. Catal. Commun. 2019, 124, 6–11. [Google Scholar] [CrossRef]
- Paniagua, M.; Cuevas, F.; Morales, G.; Melero, J.A. Sulfonic Mesostructured SBA-15 Silicas for the Solvent-Free Production of Bio-Jet Fuel Precursors via Aldol Dimerization of Levulinic Acid. ACS Sustain. Chem. Eng. 2021, 9, 5952–5962. [Google Scholar] [CrossRef]
- Albach, B.; Liz, M.V.; Prola, L.D.T.; Barbosa, R.V.; Campos, R.B.; Rampon, D.S. Eco-friendly mechanochemical intercalation of imidazole into kaolinite. J. Solid State Chem. 2020, 292, 121649. [Google Scholar] [CrossRef]
- Murtaza, S.Z.M.; Vaqueiro, P. Rapid synthesis of chalcohalides by ball milling: Preparation and characterisation of BiSI and BiSeI. J. Solid State Chem. 2020, 291, 121625. [Google Scholar] [CrossRef]
- Li, X.; Zhang, J.; Liu, B.; Liu, J.; Wang, C.; Chen, G. Hydrodeoxygenation of lignin-derived phenols to produce hydrocarbons over Ni/Al-SBA-15 prepared with different impregnants. Fuel 2019, 243, 314–321. [Google Scholar] [CrossRef]
- Xing, R.; Liu, N.; Liu, Y.; Wu, H.; Jiang, Y.; Chen, L.; He, M.; Wu, P. Novel solid acid catalysts: Sulfonic acid group-functionalized mesostructured polymers. Adv. Funct. Mater. 2007, 17, 2455–2461. [Google Scholar] [CrossRef]
- Thi Tuong Vi, T.; Kongparakul, S.; Karnjanakom, S.; Reubroycharoen, P.; Guan, G.; Chanlek, N.; Samart, C. Highly productive xylose dehydration using a sulfonic acid functionalized KIT-6 catalyst. Fuel 2019, 236, 1156–1163. [Google Scholar] [CrossRef]
- Yang, D.H.; Hur, B.Y.; He, D.P.; Yang, S.R. Effect of decomposition properties of titanium hydride on the foaming process and pore structures of Al alloy melt foam. Mater. Sci. Eng. A-Struct. Mater. Prop. Microstruct. Process. 2007, 445, 415–426. [Google Scholar] [CrossRef]
- Ellison, C.; Hoffman, J.; Shekhawat, D. Comparison of microwave and conventional heating for CO2 desorption from zeolite 13X. Int. J. Greenh. Gas Control 2021, 107, 103311. [Google Scholar] [CrossRef]
- Yu, C.; Qi, Z.; Bian, J.; Song, R.; Wang, W.; Li, C. Insight into acid-base bifunctional catalysts for microalgae liquefaction and bio-oil pyrolysis: Product characteristics, energy recovery and kinetics. J. Anal. Appl. Pyrolysis 2021, 155, 105086. [Google Scholar] [CrossRef]
- Zhang, K.; Cheung, W.H.; Valix, M. Roles of physical and chemical properties of activated carbon in the adsorption of lead ions. Chemosphere 2005, 60, 1129–1140. [Google Scholar] [CrossRef] [PubMed]
- Xia, Q.; Li, Z.; Xiao, L.; Zhang, Z.; Xi, H. Effects of loading different metal ions on an activated carbon on the desorption activation energy of dichloromethane/trichloromethane. J. Hazard. Mater. 2010, 179, 790–794. [Google Scholar] [CrossRef]
- Xue, B.; Li, Y.; Deng, L. Selective synthesis of p-xylene by alkylation of toluene with dimethyl carbonate over MgO-modified MCM-22. Catal. Commun. 2009, 10, 1609–1614. [Google Scholar] [CrossRef]
- Huang, X.; Zhang, Q.; Wang, T.; Liu, Q.; Ma, L.; Zhang, Q. Production of jet fuel intermediates from furfural and acetone by aldol condensation over MgO/NaY. J. Fuel Chem. Technol. 2012, 40, 973–978. [Google Scholar] [CrossRef]
- Li, G.; Wang, B.; Chen, B.; Resasco, D.E. Role of water in cyclopentanone self-condensation reaction catalyzed by MCM-41 functionalized with sulfonic acid groups. J. Catal. 2019, 377, 245–254. [Google Scholar] [CrossRef]
- Ngo, D.T.; Tan, Q.; Wang, B.; Resasco, D.E. Aldol Condensation of Cyclopentanone on Hydrophobized MgO. Promotional Role of Water and Changes in the Rate-Limiting Step upon Organosilane Functionalization. ACS Catal. 2019, 9, 2831–2841. [Google Scholar] [CrossRef]
- Young, Z.D.; Hanspal, S.; Davis, R.J. Aldol Condensation of Acetaldehyde over Titania, Hydroxyapatite, and Magnesia. ACS Catal. 2016, 6, 3193–3202. [Google Scholar] [CrossRef]
- Zhao, L.; An, H.; Zhao, X.; Wang, Y. TiO2-Catalyzed n-Valeraldehyde Self-Condensation Reaction Mechanism and Kinetics. ACS Catal. 2017, 7, 4451–4461. [Google Scholar] [CrossRef]
- Lu, B.; Wang, Z.; Ma, S.; Mao, S.; Chen, Z.; Wang, Y. Spatial charge separation induced new mechanism of efficient C-C coupling by forming ion-pair intermediates. Chem Catal. 2021, 1, 1449–1465. [Google Scholar] [CrossRef]
- Song, R.; Meng, X.; Yu, C.; Bian, J.; Su, J. Oil shale in-situ upgrading with natural clay-based catalysts: Enhancement of oil yield and quality. Fuel 2022, 314, 123076. [Google Scholar] [CrossRef]








| CO2 Quantity (cm3/g) | Base Amount (mmol/g) | NH3 Quantity (cm3/g) | Total Acids (mmol/g) | ||||
|---|---|---|---|---|---|---|---|
| 0–150 °C | 150–450 °C | Weak Base | Medium Base | Total Basicity | |||
| APG | 39.76 | 85.31 | 1.81 | 3.88 | 5.69 | 17.63 | 1.04 |
| SO3H-APG | 34.67 | 83.81 | 1.58 | 3.81 | 5.39 | 99.60 | 5.86 |
| SO3H-kaolin | - | - | - | - | - | 28.39 | 1.67 |
| SO3H-clin | - | - | - | - | - | 31.78 | 1.87 |
| BET Surface Area (m2/g) | Pore Volume (cm3/g) | Pore Diameter (nm) | |
|---|---|---|---|
| SO3H-APG | 127.08 | 0.0312 | 0.95 (micro)/3.83 (meso) |
| SO3H-kaolin | 117.04 | 0.1399 | 3.84 |
| SO3H-clin | 110.94 | 0.2639 | 3.82 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, X.; Su, H.; Song, R.; Su, J.; Bian, J. Solvent-Free Aldol Condensation of Cyclopentanone with Natural Clay-Based Catalysts: Origin of Activity & Selectivity. Catalysts 2023, 13, 530. https://doi.org/10.3390/catal13030530
Meng X, Su H, Song R, Su J, Bian J. Solvent-Free Aldol Condensation of Cyclopentanone with Natural Clay-Based Catalysts: Origin of Activity & Selectivity. Catalysts. 2023; 13(3):530. https://doi.org/10.3390/catal13030530
Chicago/Turabian StyleMeng, Xianglong, Hui Su, Ranran Song, Jianzheng Su, and Junjie Bian. 2023. "Solvent-Free Aldol Condensation of Cyclopentanone with Natural Clay-Based Catalysts: Origin of Activity & Selectivity" Catalysts 13, no. 3: 530. https://doi.org/10.3390/catal13030530