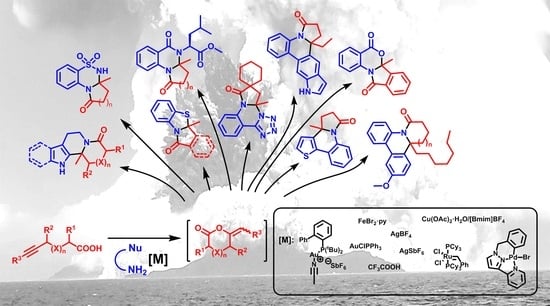
Metal-Catalyzed Cascade Reactions between Alkynoic Acids and Dinucleophiles: A Review
Abstract
:1. Introduction
2. Au Catalysts
2.1. Introduction
2.2. Initial Reports
2.3. Au-Catalyzed Reaction between Alkynoic Acids and C-, O- and N-Aminonucleophiles; Further Advances in the Field
3. Ag Catalysts
3.1. Introduction
3.2. Ag-Catalyzed Reaction between Alkynoic Acids and Aminonuclephiles
4. Cu Catalysts
4.1. Introduction
4.2. Cu-Catalyzed Cascade of 4-Pentynoic Acid and Aminonucleophiles
5. Ru Catalysts
5.1. Introduction
5.2. Cascade Reaction of Alkynoic Acids and Aminonucleophiles in the Presence of Ruthenium Carbenes
6. Pd and Fe Catalysts
6.1. Introduction
6.2. Fe-Catalyzed Cascade Reaction between Alkynoic Acids and Aminonucleophiles
6.3. Cascade Reaction of N- and O-Aminonucleophiles with Alkynoic Acids in the Presence of a Palladium Pincer Complex
7. Conclusions and Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nicolaou, K.C.; Chen, J.S. The art of total synthesis through cascade reactions. Chem. Soc. Rev. 2009, 38, 2993–3009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nicolaou, K.C.; Edmonds, D.J.; Bulger, P.G. Cascade Reactions in Total Synthesis. Angew. Chem. Int. Ed. 2006, 45, 7134–7186. [Google Scholar] [CrossRef] [PubMed]
- Hwang, E.T.; Lee, S. Multienzymatic Cascade Reactions via Enzyme Complex by Immobilization. ACS Catal. 2019, 9, 4402–4425. [Google Scholar] [CrossRef]
- Xu, Z.-J.; Liu, X.-Y.; Zhu, M.-Z.; Xu, Y.-L.; Yu, Y.; Xu, H.-R.; Cheng, A.-X.; Lou, H.-X. Photoredox-Catalyzed Cascade Reactions Involving Aryl Radical: Total Synthesis of (±)-Norascyronone A and (±)-Eudesmol. Org. Lett. 2021, 23, 9073–9077. [Google Scholar] [CrossRef]
- Hinkle, R.J.; Lewis, S.E. Atom Economical, One-Pot, Three-Reaction Cascade to Novel Tricyclic 2,4-Dihydro-1H-benzo[f]isochromenes. Org. Lett. 2013, 15, 4070–4073. [Google Scholar] [CrossRef] [Green Version]
- Buñuel, E.; Cárdenas, D.J. Towards Useful Boronates through Atom-Economical Catalyzed Cascade Reactions. Chem. Eur. J. 2018, 24, 11239–11244. [Google Scholar] [CrossRef]
- Patil, N.T.; Kavthe, R.D.; Shinde, V.S. Transition metal-catalyzed addition of C-, N- and O-nucleophiles to unactivated C-C multiple bonds. Tetrahedron 2012, 68, 8079–8146. [Google Scholar] [CrossRef]
- Suess, A.M.; Lalic, G. Copper-Catalyzed Hydrofunctionalization of Alkynes. Synlett 2016, 27, 1165–1174. [Google Scholar] [CrossRef]
- Shibuya, M.; Okamoto, M.; Fujita, S.; Abe, M.; Yamamoto, Y. Boron-Catalyzed Double Hydrofunctionalization Reactions of Unactivated Alkynes. ACS Catal. 2018, 8, 4189–4193. [Google Scholar] [CrossRef]
- Cadierno, V. Metal-Catalyzed Hydrofunctionalization Reactions of Haloalkynes. Eur. J. Inorg. Chem. 2020, 2020, 886–898. [Google Scholar] [CrossRef]
- Cheng, Z.; Guo, J.; Lu, Z. Recent advances in metal-catalysed asymmetric sequential double hydrofunctionalization of alkynes. Chem. Commun. 2020, 56, 2229–2239. [Google Scholar] [CrossRef]
- García-Fernández, P.D.; Iglesias-Sigüenza, J.; Rivero-Jerez, P.S.; Díez, E.; Gómez-Bengoa, E.; Fernández, R.; Lassaletta, J.M. Au(I)-Catalyzed Hydroalkynylation of Haloalkynes. J. Am. Chem. Soc. 2020, 142, 16082–16089. [Google Scholar] [CrossRef] [PubMed]
- Chapple, D.E.; Hoffer, M.A.; Boyle, P.D.; Blacquiere, J.M. Alkyne Hydrofunctionalization Mechanism Including an Off-Cycle Alkoxycarbene Deactivation Complex. Organometallics 2022, 41, 1532–1542. [Google Scholar] [CrossRef]
- Fang, G.; Bi, X. Silver-catalysed reactions of alkynes: Recent advances. Chem. Soc. Rev. 2015, 44, 8124–8173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, D.-W.; Gao, Y.; Shao, H.; Qiao, T.-Z.; Wang, X.; Sanchez, B.B.; Chen, J.S.; Liu, P.; Engle, K.M. Cascade CuH-catalysed conversion of alkynes into enantioenriched 1,1-disubstituted products. Nat. Catal. 2020, 3, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Corpas, J.; Mauleón, P.; Gómez Arrayás, R.; Carretero, J.C. Transition-Metal-Catalyzed Functionalization of Alkynes with Organoboron Reagents: New Trends, Mechanistic Insights, and Applications. ACS Catal. 2021, 11, 7513–7551. [Google Scholar] [CrossRef]
- Jin, S.; Liu, K.; Wang, S.; Song, Q. Enantioselective Cobalt-Catalyzed Cascade Hydrosilylation and Hydroboration of Alkynes to Access Enantioenriched 1,1-Silylboryl Alkanes. J. Am. Chem. Soc. 2021, 143, 13124–13134. [Google Scholar] [CrossRef]
- Liao, X.; Zhou, F.; Bin, Z.; Yang, Y.; You, J. Palladium-Catalyzed Cascade Dearomative Spirocyclization and C−H Annulation of Aromatic Halides with Alkynes. Org. Lett. 2021, 23, 5203–5207. [Google Scholar] [CrossRef]
- Zhang, W.-Z.; Yang, M.-W.; Yang, X.T.; Shi, L.-L.; Wang, H.-B.; Lu, X.-B. Double carboxylation of o-alkynyl acetophenone with carbon dioxide. Org. Chem. Front. 2016, 3, 217–221. [Google Scholar] [CrossRef]
- Du, Y.-L.; Zhu, Y.-F.; Han, Z.-Y. Pd(II)-Catalyzed Cycloisomerization/Dipolar Cycloaddition Cascade of N-Arylnitrone Alkynes with Olefins. J. Org. Chem. 2015, 80, 7732–7738. [Google Scholar] [CrossRef]
- Hirano, K.; Inaba, Y.; Watanabe, T.; Oishi, S.; Fujii, N.; Ohno, H. Gold-Catalyzed Intramolecular Alkyne Cycloisomerization Cascade: Direct Synthesis of Aryl-Annulated[a]carbazoles from Aniline-Substituted Diethynylarenes. Adv. Synth. Catal. 2010, 352, 368–372. [Google Scholar] [CrossRef]
- Storch, J.; Bernard, M.; Sýkora, J.; Karban, J.; Čermák, J. Intramolecular Cascade Hydroarylation/Cycloisomerization Strategy for the Synthesis of Polycyclic Aromatic and Heteroaromatic Systems. Eur. J. Org. Chem. 2013, 2013, 260–263. [Google Scholar] [CrossRef]
- Álvarez-Pérez, M.; Frutos, M.; Viso, A.; Fernández de la Pradilla, R.; de la Torre, M.C.; Sierra, M.A.; Gornitzka, H.; Hemmert, C. Gold(I)-Catalyzed Cycloisomerization–Dimerization Cascade of Benzene-Tethered 1,6-Enynes. J. Org. Chem. 2017, 82, 7546–7554. [Google Scholar] [CrossRef]
- Alonso-Marañón, L.; Sarandeses, L.A.; Martínez, M.M.; Pérez Sestelo, J. Synthesis of fused chromenes by indium(III)-catalyzed cascade hydroarylation/cycloisomerization reactions of polyyne-type aryl propargyl ethers. Org. Chem. Front. 2018, 5, 2308–2312. [Google Scholar] [CrossRef]
- Millán, R.E.; Rodríguez, J.; Sarandeses, L.A.; Gómez-Bengoa, E.; Pérez Sestelo, J. Indium(III)-Catalyzed Stereoselective Synthesis of Tricyclic Frameworks by Cascade Cycloisomerization Reactions of Aryl 1,5-Enynes. J. Org. Chem. 2021, 86, 9515–9529. [Google Scholar] [CrossRef] [PubMed]
- Nagendiran, A.; Verho, O.; Haller, C.; Johnston, E.V.; Bäckvall, J.-E. Cycloisomerization of Acetylenic Acids to γ-Alkylidene Lactones using a Palladium(II) Catalyst Supported on Amino-Functionalized Siliceous Mesocellular Foam. J. Org. Chem. 2014, 79, 1399–1405. [Google Scholar] [CrossRef] [Green Version]
- Ke, D.; Espinosa, N.A.; Ladeira, S.M.; Monot, J.; Martin-Vaca, B.; Bourissou, D. Efficient Synthesis of Unsaturated d- and e-Lactones/Lactams by Catalytic Cycloisomerization: When Pt Outperforms Pd. Adv. Synth. Catal. 2016, 358, 2324–2331. [Google Scholar] [CrossRef]
- Francos, J.; Cadierno, V. Metal-Catalyzed Intra- and Intermolecular Addition of Carboxylic Acids to Alkynes in Aqueous Media: A Review. Catalysts 2017, 7, 328. [Google Scholar] [CrossRef] [Green Version]
- Saavedra, B.; Pérez, J.M.; Rodríguez-Álvarez, M.J.; García-Álvarez, J.; Ramón, D.J. Impregnated palladium on magnetite as a water compatible catalyst for the cycloisomerization of alkynoic acid derivatives. Green Chem. 2018, 20, 2151–2157. [Google Scholar] [CrossRef] [Green Version]
- Tian, G.; Song, L.; Li, Z.; Robeyns, K.; Meervelt, L.V.; Van der Eycken, E.V. A Gold(I)-Catalyzed Hydroamination/Cycloisomerization Cascade: Concise Synthesis of (±)-seco-Antofine and (±)-Septicine. Org. Lett. 2020, 22, 8441–8445. [Google Scholar] [CrossRef]
- Skvortsova, V.I.; Bachurin, S.O.; Ustyugov, A.A.; Kukharsky, M.S.; Deikin, A.V.; Buchman, V.L.; Ninkina, N.N. Gamma-Carbolines Derivatives As Promising Agents for the Development of Pathogenic Therapy for Proteinopathy. Acta Nat. 2018, 10, 59–62. [Google Scholar] [CrossRef]
- Xu, S.-M.; Wei, L.; Shen, C.; Xiao, L.; Tao, H.-Y.; Wang, C.-J. Stereodivergent assembly of tetrahydro-γ- carbolines via synergistic catalytic asymmetric cascade reaction. Nat. Commun. 2019, 10, 5553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, J.; Dan, W.; Zhang, Y.; Wang, J. Recent developments on synthesis and biological activities of γ-carboline. Eur. J. Med. Chem. 2018, 157, 447–461. [Google Scholar] [CrossRef]
- Otto, R.; Penzis, R.; Gaube, F.; Winckler, T.; Appenroth, D.; Fleck, C.; Tränkle, C.; Lehmann, J.; Enzensperger, C. Beta and gamma carboline derivatives as potential anti-Alzheimer agents: A comparison. Eur. J. Med. Chem. 2014, 87, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Doody, R.S.; Gavrilova, S.I.; Sano, M.; Thomas, R.G.; Aisen, P.S.; Bachurin, S.O.; Seely, L.; Hung, D. Effect of dimebon on cognition, activities of daily living, behaviour, and global function in patients with mild-to-moderate alzheimer’s disease: A randomised, double-blind, placebo-controlled study. Lancet 2008, 372, 207–215. [Google Scholar] [CrossRef]
- Kalin, J.H.; Butler, K.V.; Akimova, T.; Hancock, W.W.; Kozikowski, A.P. Second-generation histone deacetylase 6 inhibitors enhance the immunosuppressive effects of Foxp3+ T-regulatory cells. J. Med. Chem. 2012, 55, 639–651. [Google Scholar] [CrossRef]
- Butler, K.V.; Kalin, J.; Brochier, C.; Vistoli, G.; Langley, B.; Kozikowski, A.P. Rational design and simple chemistry yield a superior, neuroprotective HDAC6 inhibitor, tubastatin A. J. Am. Chem. Soc. 2010, 132, 10842–10846. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Wang, Y.; Liu, W.; Liu, N.; Zhang, Y.; Dong, G.; Liu, Y.; Li, Z.; He, X.; Miao, Z.; et al. Novel carboline derivatives as potent antifungal lead compounds: Design, synthesis, and biological evaluation. ACS Med. Chem. Lett. 2014, 5, 506–511. [Google Scholar] [CrossRef] [Green Version]
- Nam, S.-J.; Kauffman, C.A.; Jensen, P.R.; Moore, C.E.; Rheingold, A.L.; Fenical, W. Actinobenzoquinoline and actinophenanthrolines A-C, unprecedented alkaloids from a marine Actinobacterium. Org. Lett. 2015, 17, 3240–3243. [Google Scholar] [CrossRef]
- Kaur, R.; Manjal, S.K.; Rawal, R.K.; Kumar, K. Recent synthetic and medicinal perspectives of tryptanthrin. Bioorg. Med. Chem. 2017, 25, 4533–4552. [Google Scholar] [CrossRef]
- Zeng, Q.; Luo, C.; Cho, J.; Lai, D.; Shen, X.; Zhang, X.; Zhou, W. Tryptanthrin exerts anti-breast cancer effects both in vitro and in vivo through modulating the inflammatory tumor microenvironment. Acta Pharm. 2021, 71, 245–266. [Google Scholar] [CrossRef]
- Jahng, Y. Progress in the studies on tryptanthrin, an alkaloid of history. Arch. Pharm. Res. 2013, 36, 517–535. [Google Scholar] [CrossRef] [PubMed]
- Moloudizargari, M.; Mikaili, P.; Aghajanshakeri, S.; Asghari, M.H.; Shayegh, J. Pharmacological and therapeutic effects of Peganum harmala and its main alkaloids. Pharmacogn. Rev. 2013, 7, 199–212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nepali, K.; Sharma, S.; Ojha, R.; Dhar, K.L. Vasicine and structurally related quinazolines. Med. Chem. Res. 2013, 22, 1–15. [Google Scholar] [CrossRef]
- Shakhidoyatov, K.M.; Elmuradov, B.Z. Tricyclic Quinazoline Alkaloids: Isolation, Synthesis, Chemical Modification, and Biological Activity. Chem. Nat. Compd. 2014, 50, 781–800. [Google Scholar] [CrossRef]
- Nepali, K.; Ojha, R.; Singh, A.; Budhiraja, A.; Bedi PM, S.; Dhar, K.L. Design, synthesis and evaluation of arylidene pyrrolo and pyrido fused quinazolones as antimicrobial agents. Lett. Drug Des. Discov. 2013, 10, 522–528. [Google Scholar] [CrossRef]
- Kshirsagar, U.A. Recent developments in the chemistry of quinazolinone alkaloids. Org. Biomol. Chem. 2015, 13, 9336–9352. [Google Scholar] [CrossRef]
- Sondhi, S.M.; Rani, R. Microwave-mediated one step synthesis of tri- and tetracyclic heterocyclic molecules. Green Chem. Lett. Rev. 2010, 3, 115–120. [Google Scholar] [CrossRef]
- Kim, Y.; Cheon, C.-H. Synthesis of quinazolinones from anthranilamides and aldehydes via metal-free aerobic oxidation in DMSO. Tetrahedron Lett. 2014, 55, 2340–2344. [Google Scholar] [CrossRef]
- Cao, L.; Huo, H.; Zeng, H.; Yu, Y.; Lu, D.; Gong, Y. One-Pot Synthesis of Quinazolin-4(3H)-ones through Anodic Oxidation and the Related Mechanistic Studies. Adv. Synth. Catal. 2018, 360, 4764–4773. [Google Scholar] [CrossRef]
- Afanasyev, O.I.; Podyacheva, E.; Rudenko, A.; Tsygankov, A.A.; Makarova, M.; Chusov, D. Redox Condensations of o-Nitrobenzaldehydes with Amines under Mild Conditions: Total Synthesis of the Vasicinone Family. J. Org. Chem. 2020, 85, 9347–9360. [Google Scholar] [CrossRef] [PubMed]
- Kshirsagar, U.A.; Argade, N.P. Facile approach to diverse range of 1,3-diaza-heterocycles: Angular/linear selectivity paradigm and a remarkable intramolecular methyl migration. Tetrahedron 2009, 65, 5244–5250. [Google Scholar] [CrossRef]
- Yang, T.; Campbell, L.; Dixon, D.J. A Au(I)-Catalyzed N-Acyl Iminium Ion. Cyclization Cascade. J. Am. Chem. Soc. 2007, 129, 12070–12071. [Google Scholar] [CrossRef] [PubMed]
- Muratore, M.E.; Holloway, C.A.; Pilling, A.W.; Storer, R.I.; Trevitt, G.; Dixon, D.J. Enantioselective Brønsted Acid-Catalyzed N-Acyliminium Cyclization Cascades. J. Am. Chem. Soc. 2009, 131, 10796–10797. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhai, Y.; Ji, X.; Liu, G.; Feng, E.; Ye, D.; Zhao, L.; Jiang, H.; Liu, H. Gold(I)-Catalyzed One-Pot Tandem Coupling/Cyclization: An Efficient Synthesis of Pyrrolo-/Pyrido[2,1-b]benzo[d][1,3]oxazin-1-ones. Adv. Synth. Catal. 2010, 352, 373–378. [Google Scholar] [CrossRef]
- Feng, E.; Zhou, Y.; Zhang, D.; Zhang, L.; Sun, H.; Jiang, H.; Liu, H. Gold(I)-Catalyzed Tandem Transformation: A Simple Approach for the Synthesis of Pyrrolo/Pyrido[2,1-a][1,3]benzoxazinones and Pyrrolo/Pyrido[2,1-a]quinazolinones. J. Org. Chem. 2010, 75, 3274–3282. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, J.; Ji, X.; Zhou, W.; Zhang, X.; Qian, W.; Jiang, H.; Liu, H. Silver- and Gold-Mediated Domino Transformation: A Strategy for Synthesizing Benzo[e]indolo[1,2-a]pyrrolo/pyrido[2,1-c][1,4]diazepine-3,9-diones. J. Org. Chem. 2011, 76, 1239–1249. [Google Scholar] [CrossRef]
- Patil, N.T.; Mutyala, A.K.; Lakshmi, P.G.V.; Gajula, B.; Sridhar, B.; Pottireddygari, G.R.; Rao, T.P. Au(I)-Catalyzed Cascade Reaction Involving Formal Double Hydroamination of Alkynes Bearing Tethered Carboxylic Groups: An Easy Access to Fused Dihydrobenzimidazoles and Tetrahydroquinazolines. J. Org. Chem. 2010, 75, 5963–5975. [Google Scholar] [CrossRef]
- Patil, N.T.; Shinde, V.S.; Sridhar, B. Relay Catalytic Branching Cascade: A Technique to Access Diverse Molecular Scaffolds. Angew. Chem. Int. Ed. 2013, 52, 2251–2255. [Google Scholar] [CrossRef]
- Feng, E.; Zhou, Y.; Zhao, F.; Chen, X.; Zhang, L.; Jiang, H.; Liu, H. Gold-catalyzed tandem reaction in water: An efficient and convenient syn-thesis of fused polycyclic indoles. Green Chem. 2012, 14, 1888–1895. [Google Scholar] [CrossRef]
- Ji, X.; Zhou, Y.; Wang, L.; Zhao, L.; Jiang, H.; Liu, H. Au(I)/Ag(I)-catalyzed cascade approach for the synthesis of benzo[4,5]imidazo[1,2-c]pyrrolo[1,2-a]quinazolinones. J. Org. Chem. 2013, 78, 4312–4318. [Google Scholar] [CrossRef]
- Qiao, J.; Jia, X.; Li, P.; Liu, X.; Zhao, J.; Zhou, Y.; Wang, J.; Liu, H.; Zhao, F. Gold-catalyzed Rapid Construction of Nitrogen-containing Heter-ocyclic Compound Library with Scaffold Diversity and Molecular Complexity. Adv. Synth. Catal. 2019, 361, 1419–1440. [Google Scholar] [CrossRef]
- Jia, X.; Li, P.; Liu, X.; Lin, J.; Chu, Y.; Yu, J.; Wang, J.; Liu, H.; Zhao, F. Green and Facile Assembly of Diverse Fused N-Heterocycles Using Gold-Catalyzed Cascade Reactions in Water. Molecules 2019, 24, 988. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naidu, S.; Reddy, S.R. Copper-catalyzed tandem reaction in ionic liquid: An efficient reusable catalyst and solvent media for the synthesis of fused poly hetero cyclic compounds. RSC Adv. 2016, 6, 62742–62746. [Google Scholar] [CrossRef]
- Naidu, S.; Reddy, S.R. A Green and Recyclable Copper and Ionic Liquid Catalytic System for the Construction of Polyheterocyclic Compounds via One-pot Tandem Coupling Reaction. ChemistrySelect 2017, 2, 1196–1201. [Google Scholar] [CrossRef]
- Alcaide, B.; Almendros, P. Non-Metathetic Behavior Patterns of Grubbs’ Carbene. Chem. Eur. J. 2003, 6, 1258–1262. [Google Scholar] [CrossRef] [PubMed]
- Alcaide, B.; Almendros, P.; Luna, A. Grubbs’ Ruthenium-Carbenes Beyond the Metathesis Reaction: Less Conventional Non-Metathetic Utility. Chem. Rev. 2009, 109, 3817–3858. [Google Scholar] [CrossRef] [PubMed]
- Takagi, R.; Yamamoto, K.; Hiraga, Y.; Kojima, S.; Abe, M. A novel non-metathetic behavior of Grubbs catalyst: Ruthenium-mediated intramolecular [3 + 2] cycloaddition of bis-1,3-dienes. J. Organomet. Chem. 2013, 723, 171–175. [Google Scholar] [CrossRef]
- KeiIio, K.; Sachimori, S.; Watanabe, T.; Fuwa, H. Ruthenium-Catalyzed Intramolecular Double Hydroalkoxylation of Internal Alkynes. Org. Lett. 2018, 20, 7851–7855. [Google Scholar] [CrossRef]
- Fuwa, H.; Sasaki, M. Exploiting Ruthenium Carbene-Catalyzed Reactions in Total Synthesis of Marine Oxacyclic Natural Products. Bull. Chem. Soc. Jpn. 2016, 89, 1403–1415. [Google Scholar] [CrossRef] [Green Version]
- Karabulut, S.; Öztürk, B.Ö.; İmamoğlu, Y. Ru-mediated selective addition reactions of carboxylic acids to internal and terminal alkynes. J. Organomet. Chem. 2010, 695, 2161–2166. [Google Scholar] [CrossRef]
- Öztürk, B.Ö.; Gürcü, D.; Şehitoğlu, S.K. Carboxylic acid addition to terminal alkynes utilizing ammonium tagged Hoveyda-Grubbs catalyst supported on magnetically separable core/shell silica: A highly reusable and air compatible catalytic system. J. Organomet. Chem. 2019, 883, 11–16. [Google Scholar] [CrossRef]
- Melis, K.; Opstal, T.; Verpoort, F. Selective Dimerisation and Addition of Carboxylic Acids to Terminal Alkynes, Catalysed by Thermolysed Grubbs’ Catalyst: A Novel Synthesis of Enynes and Vinyl Esters. Eur. J. Org. Chem. 2002, 2002, 3779–3784. [Google Scholar] [CrossRef]
- Zheng, Y.; Liu, J.; Lei, X. Ru-Catalyzed cascade reaction of α,ω-alkynoic acids and arylethylamines towards the synthesis of aryl-fused hetero-cycles. Org. Chem. Front. 2020, 7, 660–665. [Google Scholar] [CrossRef]
- Herrero, M.T.; Diaz de Sarralde, J.; SanMartin, R.; Bravo, L.; Dominguez, E. Cesium Carbonate-Promoted Hydroamidation of Alkynes: En-amides, Indoles and the Effect of Iron(III) Chloride. Adv. Synth. Catal. 2012, 354, 3054–3064. [Google Scholar] [CrossRef]
- Díaz de Sarralde, J.; Herrero, M.T.; SanMartin, R.; Domínguez, E. Procedimiento Para la Preparación de Poliheterociclos Nitrogenados Catalizado por Compuestos de Hierro. ES2549705, 30 October 2015. [Google Scholar]
- Diaz de Sarralde, J.; Astobieta, E.; Sevilla ARincón, Y.; Herrero, M.T.; Urgoitia, G.; SanMartin, R. Iron-catalyzed cascade synthesis of nitrogen polycycles from alkynoic acids and functionalized amines. Environ. Chem. Lett. 2022, 20, 3421–3427. [Google Scholar] [CrossRef]
- Conde, N.; SanMartin, R.; Herrero, M.T.; Domínguez, E. Palladium NNC Pincer Complex as an Efficient Catalyst for the Cycloisomerization of Alkynoic Acids. Adv. Synth. Catal. 2016, 358, 3283–3292. [Google Scholar] [CrossRef]
- Conde, N.; Herrero, M.T.; Urgoitia, G.; SanMartin, R. Palladium-Catalyzed Domino Cycloisomerization/Double Condensation of Acetylenic Acids with Dinucleophiles. Catalysts 2022, 12, 127. [Google Scholar] [CrossRef]
- Yao, B.; Jaccoud, C.; Wang, Q.; Zhu, J. Synergistic Effect of Palladium and Copper Catalysts: Catalytic Cyclizative Dimerization of ortho-(1-Alkynyl)benzamides Leading to Axially Chiral 1,3-Butadienes. Chem. Eur. J. 2012, 7, 5864–5868. [Google Scholar] [CrossRef]
- Nebra, N.; Monot, J.; Shaw, R.; Martin-Vaca, B.; Bourissou, D. Metal−Ligand Cooperation in the Cycloisomerization of Alkynoic Acids with Indenediide Palladium Pincer Complexes. ACS Catal. 2013, 3, 2930–2934. [Google Scholar] [CrossRef]
- Zheng, L.-L.; Bo Yin, B.; Tian, X.-C.; Yuan, M.-Y.; Li, X.-H.; Gao, F. Pd/Cu bimetallic co-catalyzed direct 2-arylation of benzoxazole with aryl chloride. Tetrahedron Lett. 2019, 60, 151316. [Google Scholar] [CrossRef]

















Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herrero, M.T.; Díaz de Sarralde, J.; Conde, N.; Herrán, A.; Urgoitia, G.; SanMartin, R. Metal-Catalyzed Cascade Reactions between Alkynoic Acids and Dinucleophiles: A Review. Catalysts 2023, 13, 495. https://doi.org/10.3390/catal13030495
Herrero MT, Díaz de Sarralde J, Conde N, Herrán A, Urgoitia G, SanMartin R. Metal-Catalyzed Cascade Reactions between Alkynoic Acids and Dinucleophiles: A Review. Catalysts. 2023; 13(3):495. https://doi.org/10.3390/catal13030495
Chicago/Turabian StyleHerrero, María Teresa, Jokin Díaz de Sarralde, Nerea Conde, Aitor Herrán, Garazi Urgoitia, and Raul SanMartin. 2023. "Metal-Catalyzed Cascade Reactions between Alkynoic Acids and Dinucleophiles: A Review" Catalysts 13, no. 3: 495. https://doi.org/10.3390/catal13030495