Enhanced Titania Photocatalyst on Magnesium Oxide Support Doped with Molybdenum
Abstract
:1. Introduction
2. Results and Discussion
2.1. Preparation of Active Photocatalyst and Its Characterization
2.2. EPR Investigation
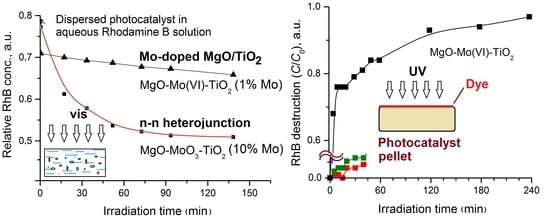
2.3. Photocatalytic Properties
3. Conclusions
4. Materials and Methods
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nakata, K.; Fujishima, A. TiO2 photocatalysis: Design and applications. J. Photochem. Photobiol. C Photochem. Rev. 2012, 13, 169–189. [Google Scholar] [CrossRef]
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 photocatalysis: Mechanisms and materials. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar] [CrossRef]
- Krut’ko, E.N.; Kulak, A.I. Heterogeneous composite titanium dioxide photocatalysts on phosphate supports. Theor. Exp. Chem. 2009, 45, 44–49. [Google Scholar] [CrossRef]
- Giannakopoulou, T.; Todorova, N.; Romanos, G.; Vaimakis, T.; Dillert, R.; Bahnemann, D.; Trapalis, C. Composite hydroxyapatite/TiO2 materials for photocatalytic oxidation of NOx. Mater. Sci. Eng. B 2012, 177, 1046–1052. [Google Scholar] [CrossRef]
- Anderson, C.; Bard, A.J. An improved photocatalyst of TiO2/SiO2 prepared by a sol-gel synthesis. J. Phys. Chem. 1995, 99, 9882–9885. [Google Scholar] [CrossRef]
- Anandan, S.; Yoon, M. Photocatalytic activities of the nano-sized TiO2-supported Y-zeolites. J. Photochem. Photobiol. C Photochem. Rev. 2003, 4, 5–18. [Google Scholar] [CrossRef]
- Fukahori, S.; Ichiura, H.; Kitaoka, T.; Tanaka, H. Capturing of bisphenol A photodecomposition intermediates by composite TiO2–zeolite sheets. Appl. Catal. B Environ. 2003, 46, 453–462. [Google Scholar] [CrossRef]
- Szczepanik, B. Photocatalytic degradation of organic contaminants over clay-TiO2 nanocomposites: A review. Appl. Clay Sci. 2017, 141, 227–239. [Google Scholar] [CrossRef]
- Ooka, C.; Yoshida, H.; Suzuki, K.; Hattori, T. Highly hydrophobic TiO2 pillared clay for photocatalytic degradation of organic compounds in water. Microporous Mesoporous Mater. 2004, 67, 143–150. [Google Scholar] [CrossRef]
- Kulak, A.I. Electrochemistry of Semiconductor Heterostructures; University Press: Minsk, Belarus, 1986; pp. 24–92. [Google Scholar]
- Papp, J.; Soled, S.; Dwight, K.; Wold, A. Surface acidity and photocatalytic activity of TiO2, WO3/TiO2, and MoO3/TiO2 photocatalysts. Chem. Mater. 1994, 6, 496–500. [Google Scholar] [CrossRef]
- Zhang, L.; Qin, M.; Yu, W.; Zhang, Q.; Xie, H.; Sun, Z.; Guo, Z. Heterostructured TiO2/WO3 nanocomposites for photocatalytic degradation of toluene under visible light. J. Electrochem. Soc. 2017, 164, H1086. [Google Scholar] [CrossRef]
- Liu, J.; Yang, S.; Wu, W.; Tian, Q.; Cui, S.; Dai, Z.; Jiang, C. 3D flowerlike α-Fe2O3@ TiO2 core–shell nanostructures: General synthesis and enhanced photocatalytic performance. ACS Sustain. Chem. Eng. 2015, 3, 2975–2984. [Google Scholar] [CrossRef]
- Li, Z.; Gao, B.; Chen, G.Z.; Mokaya, R.; Sotiropoulos, S.; Puma, G.L. Carbon nanotube/titanium dioxide (CNT/TiO2) core–shell nanocomposites with tailored shell thickness, CNT content and photocatalytic/photoelectrocatalytic properties. Appl. Catal. B Environ. 2011, 110, 50–57. [Google Scholar] [CrossRef]
- Dong, W.; Pan, F.; Xu, L.; Zheng, M.; Sow, C.H.; Wu, K.; Chen, W. Facile synthesis of CdS@TiO2 core–shell nanorods with controllable shell thickness and enhanced photocatalytic activity under visible light irradiation. Appl. Surf. Sci. 2015, 349, 279–286. [Google Scholar] [CrossRef]
- Xin, T.; Ma, M.; Zhang, H.; Gu, J.; Wang, S.; Liu, M.; Zhang, Q. A facile approach for the synthesis of magnetic separable Fe3O4@TiO2, core–shell nanocomposites as highly recyclable photocatalysts. Appl. Surf. Sci. 2014, 288, 51–59. [Google Scholar] [CrossRef]
- Kwiatkowski, M.; Chassagnon, R.; Heintz, O.; Geoffroy, N.; Skompska, M.; Bezverkhyy, I. Improvement of photocatalytic and photoelectrochemical activity of ZnO/TiO2 core/shell system through additional calcination: Insight into the mechanism. Appl. Catal. B Environ. 2017, 204, 200–208. [Google Scholar] [CrossRef]
- Pan, J.; Hühne, S.M.; Shen, H.; Xiao, L.; Born, P.; Mader, W.; Mathur, S. SnO2–TiO2 core–shell nanowire structures: Investigations on solid state reactivity and photocatalytic behavior. J. Phys. Chem. C 2011, 115, 17265–17269. [Google Scholar] [CrossRef]
- Szilagyi, I.M.; Santala, E.; Heikkilä, M.; Pore, V.; Kemell, M.; Nikitin, T.; Leskelä, M. Photocatalytic properties of WO3/TiO2 core/shell nanofibers prepared by electrospinning and atomic layer deposition. Chem. Vapor Deposit. 2013, 19, 149–155. [Google Scholar] [CrossRef]
- Li, L.; Liu, X.; Zhang, Y.; Salvador, P.A.; Rohrer, G.S. Heterostructured (Ba, Sr)TiO3/TiO2 core/shell photocatalysts: Influence of processing and structure on hydrogen production. Int. J. Hydrogen Energy 2013, 38, 6948–6959. [Google Scholar] [CrossRef]
- Hu, K.; Li, Y.; Zhao, X.; Zhao, D.; Zhao, W.; Rong, H. Photocatalytic degradation mechanism of the visible-light responsive BiVO4/TiO2 core–shell heterojunction photocatalyst. J. Inorg. Organomet. Polym. Mater. 2020, 30, 775–788. [Google Scholar] [CrossRef]
- Li, Y.; Ji, S.; Gao, Y.; Luo, H.; Kanehira, M. Core-shell VO2@TiO2 nanorods that combine thermochromic and photocatalytic properties for application as energy-saving smart coatings. Sci. Rep. 2013, 3, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Ding, S.; Xu, J.; Zhang, H.; Yang, S.; Duan, X.; Wang, S. Preparation of a pn heterojunction BiFeO3@TiO2 photocatalyst with a core–shell structure for visible-light photocatalytic degradation. Chin. J. Catal. 2017, 38, 1052–1062. [Google Scholar] [CrossRef]
- Chu, S.; Zheng, X.; Kong, F.; Wu, G.; Luo, L.; Guo, Y.; Zou, Z. Architecture of Cu2O@TiO2 core–shell heterojunction and photodegradation for 4-nitrophenol under simulated sunlight irradiation. Mater. Chem. Phys. 2011, 129, 1184–1188. [Google Scholar] [CrossRef]
- Li, Y.; Wang, L.; Li, Z.; Liu, Y.; Peng, Z.; Zhou, M.; Jin, W. Synthesis and photocatalytic property of V2O5@TiO2 core-shell microspheres towards gaseous benzene. Catal. Today 2019, 321, 164–171. [Google Scholar] [CrossRef]
- Klosek, S.; Raftery, D. Visible light driven V-doped TiO2 photocatalyst and its photooxidation of ethanol. J. Phys. Chem. B 2001, 105, 2815–2819. [Google Scholar] [CrossRef]
- Kokorin, A.I.; Pergushov, V.I.; Kulak, A.I. EPR Evidence for Dynamic Rearrangements of Vanadium Paramagnetic Centers on the Surface of V-Doped Titanium Dioxide. Catal. Lett. 2020, 150, 263–272. [Google Scholar] [CrossRef]
- Wang, S.; Bai, L.N.; Sun, H.M.; Jiang, Q.; Lian, J.S. Structure and photocatalytic property of Mo-doped TiO2 nanoparticles. Powder Technol. 2013, 244, 9–15. [Google Scholar] [CrossRef]
- Yu, J.; Yu, H.; Ao, C.H.; Lee, S.C.; Jimmy, C.Y.; Ho, W. Preparation, characterization and photocatalytic activity of in situ Fe-doped TiO2 thin films. Thin Solid Films 2006, 496, 273–280. [Google Scholar] [CrossRef]
- Kokorin, A.I.; Amal, R.; Teoh, W.Y.; Kulak, A.I. Studies of nanosized iron-doped TiO2 photocatalysts by spectroscopic methods. Appl. Magn. Reson. 2017, 48, 447–459. [Google Scholar] [CrossRef]
- Wang, C.; Shi, H.; Li, Y. Synthesis and characterization of natural zeolite supported Cr-doped TiO2 photocatalysts. Appl. Surf. Sci. 2012, 258, 4328–4333. [Google Scholar] [CrossRef]
- Zaleska, A. Doped-TiO2: A review. Recent Pat. Eng. 2008, 2, 157–164. [Google Scholar] [CrossRef]
- Akpan, U.G.; Hameed, B.H. The advancements in sol–gel method of doped-TiO2 photocatalysts. Appl. Catal. A General 2010, 375, 1–11. [Google Scholar] [CrossRef]
- Basavarajappa, P.S.; Patil, S.B.; Ganganagappa, N.; Reddy, K.R.; Raghu, A.V.; Reddy, C.V. Recent progress in metal-doped TiO2, non-metal doped/codoped TiO2 and TiO2 nanostructured hybrids for enhanced photocatalysis. Int. J. Hydrogen Energy 2020, 45, 7764–7778. [Google Scholar] [CrossRef]
- Wang, Z.; Lin, Z.; Shen, S.; Zhong, W.; Cao, S. Advances in designing heterojunction photocatalytic materials. Chin. J. Catal. 2021, 42, 710–730. [Google Scholar] [CrossRef]
- Liao, G.; Li, C.; Liu, S.Y.; Fang, B.; Yang, H. Emerging frontiers of Z-scheme photocatalytic systems. Trends Chem. 2022, 4, 111–127. [Google Scholar] [CrossRef]
- Ren, G.; Han, H.; Wang, Y.; Liu, S.; Zhao, J.; Meng, X.; Li, Z. Recent advances of photocatalytic application in water treatment: A review. Nanomaterials 2021, 11, 1804. [Google Scholar] [CrossRef]
- Wang, H.; Li, X.; Zhao, X.; Li, C.; Song, X.; Zhang, P.; Huo, P. A review on heterogeneous photocatalysis for environmental remediation: From semiconductors to modification strategies. Chin. J. Catal. 2022, 43, 178–214. [Google Scholar] [CrossRef]
- Fang, M.; Tan, X.; Liu, Z.; Hu, B.; Wang, X. Recent progress on metal-enhanced photocatalysis: A review on the mechanism. Research 2021, 2021, 9794329. [Google Scholar] [CrossRef]
- Weng, B.; Qi, M.Y.; Han, C.; Tang, Z.R.; Xu, Y.J. Photocorrosion inhibition of semiconductor-based photocatalysts: Basic principle, current development, and future perspective. ACS Catal. 2019, 9, 4642–4687. [Google Scholar] [CrossRef]
- Karim, A.V.; Krishnan, S.; Shriwastav, A. An overview of heterogeneous photocatalysis for the degradation of organic compounds: A special emphasis on photocorrosion and reusability. J. Indian Chem. Soc. 2022, 99, 100480. [Google Scholar] [CrossRef]
- Khan, M.; Xu, J.; Cao, W.; Liu, Z.K. Mo-doped TiO2 with enhanced visible light photocatalytic activity: A combined experimental and theoretical study. J. Nanosci. Nanotechnol. 2014, 14, 6865–6871. [Google Scholar] [CrossRef] [PubMed]
- Stengl, V.; Bakardjieva, S. Molybdenum-doped anatase and its extraordinary photocatalytic activity in the degradation of orange II in the UV and vis regions. J. Phys. Chem. C 2010, 114, 19308–19317. [Google Scholar] [CrossRef]
- Fisher, L.; Ostovapour, S.; Kelly, P.; Whitehead, K.A.; Cooke, K.; Storgårds, E.; Verran, J. Molybdenum doped titanium dioxide photocatalytic coatings for use as hygienic surfaces: The effect of soiling on antimicrobial activity. Biofouling 2014, 30, 911–919. [Google Scholar] [CrossRef] [PubMed]
- Alnaggar, G.; Alkanad, K.; Chandrashekar, S.S.G.; Bajiri, M.A.; Drmosh, Q.A.; Krishnappagowda, L.N.; Ananda, S. Rational design of a 2D TiO2–MoO3 step-scheme heterostructure for boosted photocatalytic overall water splitting. New J. Chem. 2022, 46, 9629–9640. [Google Scholar] [CrossRef]
- Bai, S.; Liu, H.; Sun, J.; Tian, Y.; Chen, S.; Song, J.; Liu, C.C. Improvement of TiO2 photocatalytic properties under visible light by WO3/TiO2 and MoO3/TiO2 composites. Appl. Surf. Sci. 2015, 338, 61–68. [Google Scholar] [CrossRef]
- Kong, F.; Huang, L.; Luo, L.; Chu, S.; Wang, Y.; Zou, Z. Synthesis and characterization of visible light driven mesoporous nano-photocatalyst MoO3/TiO2. J. Nanosci. Nanotechnol. 2012, 12, 1931–1937. [Google Scholar] [CrossRef]
- Odhiambo, V.O.; Le Ba, T.; Kónya, Z.; Cserháti, C.; Erdélyi, Z.; Naomi, M.C.; Szilágyi, I.M. Preparation of TiO2–MoO3 composite nanofibers by water-based electrospinning process and their application in photocatalysis. Mater. Sci. Semicond. Process. 2022, 147, 106699. [Google Scholar] [CrossRef]
- Yang, Y.; Li, X.J.; Chen, J.T.; Wang, L.Y. Effect of doping mode on the photocatalytic activities of Mo/TiO2. J. Photochem. Photobiol. A Chem. 2004, 163, 517–522. [Google Scholar] [CrossRef]
- Natori, H.; Kobayashi, K.; Takahashi, M. Fabrication and photocatalytic activity of TiO2/MoO3 particulate films. J. Oleo Sci. 2009, 58, 203–211. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, Y.; Ngaotrakanwiwat, P.; Tatsuma, T. Energy storage TiO2–MoO3 photocatalysts. Electrochim. Acta 2004, 49, 2025–2029. [Google Scholar] [CrossRef]
- Song, K.Y.; Park, M.K.; Kwon, Y.T.; Lee, H.W.; Chung, W.J.; Lee, W.I. Preparation of transparent particulate MoO3/TiO2 and WO3/TiO2 films and their photocatalytic properties. Chem. Mater. 2001, 13, 2349–2355. [Google Scholar] [CrossRef]
- Devi, L.G.; Murthy, B.N.; Kumar, S.G. Photocatalytic activity of V5+, Mo6+ and Th4+ doped polycrystalline TiO2 for the degradation of chlorpyrifos under UV/solar light. J. Mol. Catal. A Chem. 2009, 308, 174–181. [Google Scholar] [CrossRef]
- Elder, S.H.; Cot, F.M.; Su, Y.; Heald, S.M.; Tyryshkin, A.M.; Bowman, M.K.; Blake, D.M. The discovery and study of nanocrystalline TiO2-(MoO3) core-shell materials. JACS 2000, 122, 5138–5146. [Google Scholar] [CrossRef]
- Liu, L.; Zhao, C.; Zhao, H.; Pitts, D.; Li, Y. Porous microspheres of MgO-patched TiO2 for CO2 photoreduction with H2O vapor: Temperature-dependent activity and stability. Chem. Commun. 2013, 49, 3664–3666. [Google Scholar] [CrossRef] [PubMed]
- Bandara, J.; Hadapangoda, C.C.; Jayasekera, W.G. TiO2/MgO composite photocatalyst: The role of MgO in photoinduced charge carrier separation. Appl. Catal. B Environ. 2004, 50, 83–88. [Google Scholar] [CrossRef]
- Shrestha, K.M.; Sorensen, C.M.; Klabunde, K.J. MgO–TiO2 mixed oxide nanoparticles: Comparison of flame synthesis versus aerogel method; characterization, and photocatalytic activities. J. Mater. Res. 2013, 28, 431–439. [Google Scholar] [CrossRef]
- Wu, S.; Han, H.; Tai, Q.; Zhang, J.; Xu, S.; Zhou, C.; Zhao, X.Z. Enhancement in dye-sensitized solar cells based on MgO-coated TiO2 electrodes by reactive DC magnetron sputtering. Nanotechnology 2008, 19, 215704. [Google Scholar] [CrossRef]
- Raman, C.V. The vibrations of the MgO crystal structure and its infra-red absorption spectrum. Proc. Indian Acad. Sci. 1961, A54, 205–222. [Google Scholar] [CrossRef]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds, Part B: Applications in Coordination, Organometallic, and Bioinorganic Chemistry; John Wiley & Sons: Hoboken, NJ, USA, 2009; 400 p. [Google Scholar]
- Benesi, H.A. Infrared spectrum of Mg(OH)2. J. Chem. Phys. 1959, 30, 852. [Google Scholar] [CrossRef]
- Mestl, G.; Verbruggen, N.F.D.; Knozinger, H. Mechanically activated MoO3. 2 Characterization of defect structures. Langmuir 1995, 11, 3035–3041. [Google Scholar] [CrossRef]
- Mestl, G.; Verbruggen, N.F.D.; Bosch, E.; Knozinger, H. Mechanically Activated MoO3. 5. Redox behavior. Langmuir 1996, 12, 2961–2968. [Google Scholar] [CrossRef]
- Streletskii, A.N.; Sivak, M.V.; Dolgoborodov, A.Y. Nature of high reactivity of metal/solid oxidizer nanocomposites prepared by mechanoactivation: A review. J. Mater. Sci. 2017, 52, 11810–11825. [Google Scholar] [CrossRef]
- Konstantinova, E.A.; Kokorin, A.I.; Minnekhanov, A.A.; Sviridova, T.V.; Sviridov, D.V. EPR Study of Photoexcited Charge Carrier Behavior in TiO2/MoO3 and TiO2/MoO3:V2O5 Photocatalysts. Catal. Lett. 2019, 149, 2256–2267. [Google Scholar] [CrossRef]
- Logvinovich, A.S.; Sviridova, T.V.; Konstantinova, E.A.; Kokorin, A.I.; Sviridov, D.V. Solvothermally-derived MoO3-benzotriazole hybrid structures for nanocontainer depot systems. New J. Chem. 2020, 44, 11131–11136. [Google Scholar] [CrossRef]
- Al’tshuler, S.A.; Kozyrev, B.M. Elektron Paramagnetic Resonance in Compounds of Transition Elements; J. Wiley & Sons: New York, NY, USA, 1974. [Google Scholar]
- Kokorin, A.I. Electron spin resonance of nanostructured oxide semiconductors. In Chemical Physics of Nanostructured Semiconductors; Kokorin, A., Bahnemann, D., Eds.; VSP-Brill Academic Publishers: Boston, MA, USA, 2003; p. 203. [Google Scholar]
- Weil, J.A.; Bolton, J.R. Relaxation Times, Linewidths and Spin Kinetic Phenomena. In Electron Paramagnetic Resonance; John Wiley & Sons: New York, NY, USA, 2006; pp. 301–356. [Google Scholar]
- Labanowska, M. Paramagnetic defects in MoO3—revisited. Phys. Chem. Chem. Phys. 1999, 1, 5385–5392. [Google Scholar] [CrossRef]
- Nova, I.; Lietti, L.; Casagrande, L.; Dall’Acqua, L.; Giamello, E.; Forzatti, P. Characterization and Reactivity of TiO2-Supported MoO3 de-NOx SCR Catalysts. Appl. Catal. B 1998, 17, 245–258. [Google Scholar] [CrossRef]
- Dall’Acqua, L.; Nova, I.; Lietti, L.; Ramis, G.; Busca, G.; Giamello, E. Spectroscopic Characterisation of MoO3/TiO2 de-NOx SCR Catalysts: Redox and Coordination Properties. Phys. Chem. Chem. Phys. 2000, 2, 4991–4998. [Google Scholar] [CrossRef]
- Dyrek, K.; Labanowska, M. Electron paramagnetic resonance investigation of the paramagnetic centres in polycrystalline MoO3. J. Chem. Soc. Faraday Trans. 1991, 87, 1003–1009. [Google Scholar] [CrossRef]
- Eaton, G.R.; Eaton, S.S.; Barr, D.P.; Weber, R.T. Quantitative EPR; Springer: Vienna, Austria, 2010. [Google Scholar]
- Liu, Y.; Feng, P.; Wang, Z.; Jiao, X.; Akhtar, F. Novel fabrication and enhanced photocatalytic MB degradation of hierarchical porous monoliths of MoO3 nanoplates. Sci. Rep. 2017, 7, 1–12. [Google Scholar]
- Hu, H.; Deng, C.; Xu, J.; Zhang, K.; Sun, M. Metastable h-MoO3 and stable α-MoO3 microstructures: Controllable synthesis, growth mechanism and their enhanced photocatalytic activity. J. Exp. Nanosci. 2015, 10, 1336–1346. [Google Scholar] [CrossRef]
- Nagpal, M.; Kakkar, R. Facile synthesis of mesoporous magnesium oxide–graphene oxide composite for efficient and highly selective adsorption of hazardous anionic dyes. Res. Chem. Intermed. 2020, 46, 2497–2521. [Google Scholar] [CrossRef]
- Kuska, H.A.; Rogers, M.T. ESR of First Row Transition Metal Complex Ions; Interscience: New York, NY, USA, 1968; Mir: Moscow, Russia, 1970. [Google Scholar]
- Vorobiev, A.K.; Chumakova, N.A. Simulation of rigidlimit and slow-motion EPR spectra for extraction of quantitative dynamic and orientational information. In Nitroxides—Theory, Experiment and Applications; Kokorin, A.I., Ed.; InTech Publ: Riecka, Slovakia, 2012; pp. 57–112. [Google Scholar]









| Composite | [Mo], wt.% | r, nm | Sa m2/g | Vp, cm3/g | Dp, nm | k1, min−1 (UV) | k1, min−1 (vis) |
|---|---|---|---|---|---|---|---|
| Pristine MgO | 0 | 43.6 | 42 | 0.11 | 6.14 | N/A | N/A |
| MgO-TiO2 | 0 | 10.1 | 50 | 0.18 | 10.9 | 0.028 | <0.001 |
| MgO-Mo(VI) | 1.0 | 9.4 | 208 | 0.45 | 5.46 | N/A | N/A |
| MgO-Mo(VI)-TiO2 | 0.5 | 12.5 | 191 | 0.33 | 5.05 | 0.035 | <0.001 |
| MgO-Mo(VI)-TiO2 | 1.0 | 9.4 | 63 | 0.19 | 7.75 | 0.037 | <0.001 |
| MgO-Mo(VI)-TiO2 | 2.0 | 9.3 | 210 | 0.37 | 5.12 | 0.031 | 0.002 |
| MgO-Mo(VI)-TiO2; MgO-MoO3-TiO2 | 5.0 | 9.7 | 193 | 0.29 | 4.01 | 0.020 | 0.003 |
| MgO-MoO3-TiO2 | 10.0 | 10.9 | 110 | 0.22 | 3.77 | 0.019 | 0.012 |
| MgO-MoO3-TiO2 | 20.0 | 10.4 | 76 | 0.21 | 3.15 | 0.014 | 0.013 |
| Sample | g⊥ (g1) | g|| (g2) | g|| (g3)g3 | Ref. |
|---|---|---|---|---|
| MgO-MoO3 | 1.925 | - | 1.840 | This work |
| MoO3 | 1.923 | 1.912 | 1.839 | This work |
| MoO3/TiO2 | 1.9237 | - | 1.8755 | [71] |
| MoO3/TiO2 | 1.944 | 1.844 | 1.839 | [72] |
| MoO3/TiO2 | 1.916 | - | 1.819 | [65] |
| MoO3 | 1.96 | 1.94 | 1.87 | [62,63] |
| MoO3 | 1.954 | 1.941 | 1.865 | [73] |
| MoO3 | 1.936 | - | 1.899 | [73] |
| MoO3 | 1.957 | 1.943 | 1.864 | [70] |
| MoO3 | 1.933 | - | 1.910 | [70] |
| Sample | Mo/Initial | Mo/300° | Mo/400° |
|---|---|---|---|
| [M5+], spin/g | 2.6 × 1017 | 2.1 × 1018 | 1.6 × 1018 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kulak, A.; Kokorin, A. Enhanced Titania Photocatalyst on Magnesium Oxide Support Doped with Molybdenum. Catalysts 2023, 13, 454. https://doi.org/10.3390/catal13030454
Kulak A, Kokorin A. Enhanced Titania Photocatalyst on Magnesium Oxide Support Doped with Molybdenum. Catalysts. 2023; 13(3):454. https://doi.org/10.3390/catal13030454
Chicago/Turabian StyleKulak, Anatoly, and Alexander Kokorin. 2023. "Enhanced Titania Photocatalyst on Magnesium Oxide Support Doped with Molybdenum" Catalysts 13, no. 3: 454. https://doi.org/10.3390/catal13030454