Effect of the Calcination Temperature of LaNiO3 on the Structural Properties and Reaction Performance of Catalysts in the Steam Reforming of Methane
Abstract
:1. Introduction
2. Materials and Methods
2.1. Catalysts Preparation
2.2. Catalysts Characterization
2.3. Catalytic Performance Evaluation
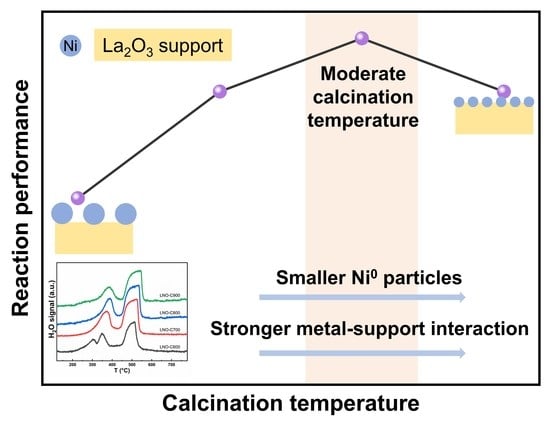
3. Results and Discussion
3.1. Formation of LaNiO3 upon Calcination of the Gel Precursor in Air
3.1.1. The Thermal Decomposition Analysis of the Dry Gel
3.1.2. The Crystallization of LaNiO3 Crystalline Phase
3.2. Physical Properties of Calcinated LaNiO3 Oxide
3.3. Reducibility of Calcinated LaNiO3 Oxides
3.4. Characterization of Reduced Catalysts
3.5. Reaction Performance of Reduced LaNiO3 Samples
3.6. Spent Catalysts Characterization
3.6.1. Effect of S/C on Crystalline Phase
3.6.2. Resistance to Carbon Deposition
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sehested, J. Four challenges for nickel steam-reforming catalysts. Catal. Today 2006, 111, 103–110. [Google Scholar] [CrossRef]
- Meloni, E.; Martino, M.; Palma, V. A Short Review on Ni Based Catalysts and Related Engineering Issues for Methane Steam Reforming. Catalysts 2020, 10, 352. [Google Scholar] [CrossRef]
- Pashchenko, D.; Makarov, I. Carbon deposition in steam methane reforming over a Ni-based catalyst: Experimental and thermodynamic analysis. Energy 2021, 222, 119993. [Google Scholar] [CrossRef]
- Garba, Z.N.; Zhou, W.; Zhang, M.; Yuan, Z. A review on the preparation, characterization and potential application of perovskites as adsorbents for wastewater treatment. Chemosphere 2020, 244, 125474. [Google Scholar] [CrossRef] [PubMed]
- Fierro, J.L.G. Hydrogenation of Carbon Oxides over Perovskite-Type Oxides. Catal. Rev. 1992, 34, 321–336. [Google Scholar] [CrossRef]
- Singh, U.G.; Li, J.; Bennett, J.W.; Rappe, A.M.; Seshadri, R.; Scott, S.L. A Pd-doped perovskite catalyst, BaCe1−xPdxO3−δ, for CO oxidation. J. Catal. 2007, 249, 349–358. [Google Scholar] [CrossRef]
- Seiyama, T. Total Oxidation of Hydrocarbons on Perovskite Oxides. Catal. Rev. 1992, 34, 281–300. [Google Scholar] [CrossRef]
- Zhang, R.; Alamdari, H.; Kaliaguine, S. SO2 poisoning of LaFe0.8Cu0.2O3 perovskite prepared by reactive grinding during NO reduction by C3H6. Appl. Catal. A Gen. 2008, 340, 140–151. [Google Scholar] [CrossRef]
- Zhu, J.; Thomas, A. Perovskite-type mixed oxides as catalytic material for NO removal. Appl. Catal. B Environ. 2009, 92, 225–233. [Google Scholar] [CrossRef]
- Zhu, J.; Li, H.; Zhong, L.; Xiao, P.; Xu, X.; Yang, X.; Zhao, Z.; Li, J. Perovskite Oxides: Preparation, Characterizations, and Applications in Heterogeneous Catalysis. ACS Catal. 2014, 4, 2917–2940. [Google Scholar] [CrossRef]
- Shao, Z.; Zhou, W.; Zhu, Z. Advanced synthesis of materials for intermediate-temperature solid oxide fuel cells. Prog. Mater. Sci. 2012, 57, 804–874. [Google Scholar] [CrossRef]
- Royer, S.; Duprez, D.; Can, F.; Courtois, X.; Batiot-Dupeyrat, C.; Laassiri, S.; Alamdari, H. Perovskites as Substitutes of Noble Metals for Heterogeneous Catalysis: Dream or Reality. Chem. Rev. 2014, 114, 10292–10368. [Google Scholar] [CrossRef]
- Maneerung, T.; Hidajat, K.; Kawi, S. LaNiO3 perovskite catalyst precursor for rapid decomposition of methane: Influence of temperature and presence of H2 in feed stream. Catal. Today 2011, 171, 24–35. [Google Scholar] [CrossRef]
- Batiot-Dupeyrat, C.; Valderrama, G.; Meneses, A.; Martinez, F.; Barrault, J.; Tatibouët, J.M. Pulse study of CO2 reforming of methane over LaNiO3. Appl. Catal. A Gen. 2003, 248, 143–151. [Google Scholar] [CrossRef]
- Choi, S.O.; Moon, S.H. Performance of La1−xCexFe0.7Ni0.3O3 perovskite catalysts for methane steam reforming. Catal. Today 2009, 146, 148–153. [Google Scholar] [CrossRef]
- Valderrama, G.; Kiennemann, A.; Goldwasser, M.R. La-Sr-Ni-Co-O based perovskite-type solid solutions as catalyst precursors in the CO2 reforming of methane. J. Power Sources 2010, 195, 1765–1771. [Google Scholar] [CrossRef]
- Villoria, J.-A.; Mota, N.; Al-Sayari, S.; Álvarez-Galván, M.-C. Perovskites as catalysts in the reforming of hydrocarbons: A review. Micro Nanosyst. 2012, 4, 231–252. [Google Scholar] [CrossRef]
- Yang, Q.; Liu, G.; Liu, Y. Perovskite-Type Oxides as the Catalyst Precursors for Preparing Supported Metallic Nanocatalysts: A Review. Ind. Eng. Chem. Res. 2018, 57, 1–17. [Google Scholar] [CrossRef]
- Bian, Z.; Wang, Z.; Jiang, B.; Hongmanorom, P.; Zhong, W.; Kawi, S. A review on perovskite catalysts for reforming of methane to hydrogen production. Renew. Sustain. Energy Rev. 2020, 134, 110291. [Google Scholar] [CrossRef]
- Bhattar, S.; Abedin, M.A.; Kanitkar, S.; Spivey, J.J. A review on dry reforming of methane over perovskite derived catalysts. Catal. Today 2021, 365, 2–23. [Google Scholar] [CrossRef]
- Pena, M.; Fierro, J. Chemical structures and performance of perovskite oxides. Chem. Rev. 2001, 101, 1981–2018. [Google Scholar] [CrossRef] [PubMed]
- Arandiyan, H.; Li, J.; Ma, L.; Hashemnejad, S.M.; Mirzaei, M.Z.; Chen, J.; Chang, H.; Liu, C.; Wang, C.; Chen, L. Methane reforming to syngas over LaNixFe1−xO3 (0 ≤ x ≤ 1) mixed-oxide perovskites in the presence of CO2 and O2. J. Ind. Eng. Chem. 2012, 18, 2103–2114. [Google Scholar] [CrossRef]
- Jahangiri, A.; Aghabozorg, H.; Pahlavanzadeh, H. Effects of Fe substitutions by Ni in La–Ni–O perovskite-type oxides in reforming of methane with CO2 and O2. Int. J. Hydrog. Energy 2013, 38, 10407–10416. [Google Scholar] [CrossRef]
- Yang, E.-h.; Noh, Y.-s.; Ramesh, S.; Lim, S.S.; Moon, D.J. The effect of promoters in La0.9M0.1Ni0.5Fe0.5O3 (M = Sr, Ca) perovskite catalysts on dry reforming of methane. Fuel Process. Technol. 2015, 134, 404–413. [Google Scholar] [CrossRef]
- Tsoukalou, A.; Imtiaz, Q.; Kim, S.M.; Abdala, P.M.; Yoon, S.; Müller, C.R. Dry-reforming of methane over bimetallic Ni–M/La2O3 (M = Co, Fe): The effect of the rate of La2O2CO3 formation and phase stability on the catalytic activity and stability. J. Catal. 2016, 343, 208–214. [Google Scholar] [CrossRef]
- Wang, M.; Zhao, T.; Dong, X.; Li, M.; Wang, H. Effects of Ce substitution at the A-site of LaNi0.5Fe0.5O3 perovskite on the enhanced catalytic activity for dry reforming of methane. Appl. Catal. B Environ. 2018, 224, 214–221. [Google Scholar] [CrossRef]
- Komarala, E.P.; Komissarov, I.; Rosen, B.A. Effect of Fe and Mn Substitution in LaNiO3 on Exsolution, Activity, and Stability for Methane Dry Reforming. Catalysts 2019, 10, 27. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, C.; Chen, S.; Liu, Y. Co–Ni bimetal catalyst supported on perovskite-type oxide for steam reforming of ethanol to produce hydrogen. Int. J. Hydrog. Energy 2014, 39, 5644–5652. [Google Scholar] [CrossRef]
- Liu, F.; Qu, Y.; Yue, Y.; Liu, G.; Liu, Y. Nano bimetallic alloy of Ni–Co obtained from LaCoxNi1− xO 3 and its catalytic performance for steam reforming of ethanol. RSC Adv. 2015, 5, 16837–16846. [Google Scholar] [CrossRef]
- Zhao, L.; Han, T.; Wang, H.; Zhang, L.; Liu, Y. Ni-Co alloy catalyst from LaNi1−xCoxO3 perovskite supported on zirconia for steam reforming of ethanol. Appl. Catal. B Environ. 2016, 187, 19–29. [Google Scholar] [CrossRef]
- Kim, W.Y.; Jang, J.S.; Ra, E.C.; Kim, K.Y.; Kim, E.H.; Lee, J.S. Reduced perovskite LaNiO3 catalysts modified with Co and Mn for low coke formation in dry reforming of methane. Appl. Catal. A Gen. 2019, 575, 198–203. [Google Scholar] [CrossRef]
- Kwon, O.; Huang, R.; Cao, T.; Vohs, J.M.; Gorte, R.J. Dry reforming of methane over Ni supported on LaMnO3 thin films. Catal. Today 2021, 382, 142–147. [Google Scholar] [CrossRef]
- Kwon, O.; Foucher, A.C.; Huang, R.; Stach, E.A.; Vohs, J.M.; Gorte, R.J. Evidence for redispersion of Ni on LaMnO3 films following high-temperature oxidation. J. Catal. 2022, 407, 213–220. [Google Scholar] [CrossRef]
- Nishihata, Y.; Mizuki, J.; Akao, T.; Tanaka, H.; Uenishi, M.; Kimura, M.; Okamoto, T.; Hamada, N. Self-regeneration of a Pd-perovskite catalyst for automotive emissions control. Nature 2002, 418, 164–167. [Google Scholar] [CrossRef]
- Neagu, D.; Oh, T.-S.; Miller, D.N.; Ménard, H.; Bukhari, S.M.; Gamble, S.R.; Gorte, R.J.; Vohs, J.M.; Irvine, J.T.S. Nano-socketed nickel particles with enhanced coking resistance grown in situ by redox exsolution. Nat. Commun. 2015, 6, 8120. [Google Scholar] [CrossRef]
- Lin, C.; Foucher, A.C.; Stach, E.A.; Gorte, R.J. A Thermodynamic Investigation of Ni on Thin-Film Titanates (ATiO3). Inorganics 2020, 8, 69. [Google Scholar] [CrossRef]
- Mao, X.; Foucher, A.C.; Stach, E.A.; Gorte, R.J. Changes in Ni-NiO equilibrium due to LaFeO3 and the effect on dry reforming of CH4. J. Catal. 2020, 381, 561–569. [Google Scholar] [CrossRef]
- Pereñiguez, R.; Gonzalez-delaCruz, V.M.; Caballero, A.; Holgado, J.P. LaNiO3 as a precursor of Ni/La2O3 for CO2 reforming of CH4: Effect of the presence of an amorphous NiO phase. Appl. Catal. B Environ. 2012, 123–124, 324–332. [Google Scholar]
- Rida, K.; Peña, M.A.; Sastre, E.; Martinez-Arias, A. Effect of calcination temperature on structural properties and catalytic activity in oxidation reactions of LaNiO3 perovskite prepared by Pechini method. J. Rare Earths 2012, 30, 210–216. [Google Scholar] [CrossRef]
- Haynes, D.J.; Shekhawat, D.; Berry, D.A.; Zondlo, J.; Roy, A.; Spivey, J.J. Characterization of calcination temperature on a Ni-substituted lanthanum-strontium-zirconate pyrochlore. Ceram. Int. 2017, 43, 16744–16752. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, Z.; Das, S.; Xi, S.; Kawi, S. LaNiO3 as a precursor of Ni/La2O3 for reverse water-gas shift in DBD plasma: Effect of calcination temperature. Energy Convers. Manag. 2020, 206, 112475. [Google Scholar] [CrossRef]
- González-Varela, D.; Araiza, D.G.; Díaz, G.; Pfeiffer, H. LaNiO3 Perovskite Synthesis through the EDTA–Citrate Complexing Method and Its Application to CO Oxidation. Catalysts 2022, 12, 57. [Google Scholar] [CrossRef]
- Pechini, M. Method of Preparing Lead and Alkaline Earth Titanates and Niobates and Coating Method Using the Same to Form a Capacitor. US Patent No. 3330697A, 11 July 1967. [Google Scholar]
- Djani, F.; Omari, M.; Martínez-Arias, A. Synthesis, characterization and catalytic properties of La(Ni,Fe)O3–NiO nanocomposites. J. Sol.-Gel Sci. Technol. 2015, 78, 1–10. [Google Scholar] [CrossRef]
- Devi, P.S.; Rao, M.S. Study of the thermal decomposition of lanthanum and chromium citrate hydrates. J. Anal. Appl. Pyrolysis 1992, 22, 187–195. [Google Scholar] [CrossRef]
- Wang, H.; Zhu, Y.; Liu, P.; Yao, W. Preparation of nanosized perovskite LaNiO3 powder via amorphous heteronuclear complex precursor. J. Mater. Sci. 2003, 38, 1939–1943. [Google Scholar] [CrossRef]
- Fernandes, J.D.G.; Melo, D.M.A.; Zinner, L.B.; Salustiano, C.M.; Silva, Z.R.; Martinelli, A.E.; Cerqueira, M.; Alves Júnior, C.; Longo, E.; Bernardi, M.I.B. Low-temperature synthesis of single-phase crystalline LaNiO3 perovskite via Pechini method. Mater. Lett. 2002, 53, 122–125. [Google Scholar] [CrossRef]
- Fjellvåg, H.; Hansteen, O.H.; Tilset, B.G.; Olafsen, A.; Sakai, N.; Seim, H. Thermal analysis as an aid in the synthesis of non-stoichiometric perovskite type oxides. Thermochim. Acta 1995, 256, 75–89. [Google Scholar] [CrossRef]
- Dreyer, M.; Krebs, M.; Najafishirtari, S.; Rabe, A.; Friedel Ortega, K.; Behrens, M. The Effect of Co Incorporation on the CO Oxidation Activity of LaFe1−xCoxO3 Perovskites. Catalysts 2021, 11, 550. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, J.; Yang, X.; Lu, L.; Wang, X. Preparation and characterization of LaNiO3 nanocrystals. Mater. Res. Bull. 2006, 41, 1565–1570. [Google Scholar] [CrossRef]
- Le, N.T.H.; Calderón-Moreno, J.M.; Popa, M.; Crespo, D.; Van Hong, L.; Phuc, N.X. LaNiO3 nanopowder prepared by an ‘amorphous citrate’ route. J. Eur. Ceram. Soc. 2006, 26, 403–407. [Google Scholar] [CrossRef]
- Valderrama, G.; Goldwasser, M.R.; Navarro, C.U.D.; Tatibouët, J.M.; Barrault, J.; Batiot-Dupeyrat, C.; Martínez, F. Dry reforming of methane over Ni perovskite type oxides. Catal. Today 2005, 107–108, 785–791. [Google Scholar]
- Santos, D.B.L.; Noronha, F.B.; Hori, C.E. Bi-reforming of methane for hydrogen production using LaNiO3/CexZr1-xO2 as precursor material. Int. J. Hydrog. Energy 2020, 45, 13947–13959. [Google Scholar] [CrossRef]
- Ramon, A.P.; Li, X.; Clark, A.H.; Safonova, O.V.; Marcos, F.C.; Assaf, E.M.; van Bokhoven, J.A.; Artiglia, L.; Assaf, J.M. In situ study of low-temperature dry reforming of methane over La2Ce2O7 and LaNiO3 mixed oxides. Appl. Catal. B Environ. 2022, 315, 121528. [Google Scholar] [CrossRef]
- Ruckenstein, E.; Hu, Y. Interactions between Ni and La2O3 in Ni/La2O3 Catalysts Prepared Using Different Ni Precursors. J. Catal. 1996, 161, 55–61. [Google Scholar] [CrossRef]
- Cesteros, Y.; Salagre, P.; Medina, F.; Sueiras, J.E. Preparation and Characterization of Several High-Area NiAl2O4 Spinels. Study of Their Reducibility. Chem. Mater. 2000, 12, 331–335. [Google Scholar] [CrossRef]
- Courson, C.; Udron, L.; Świerczyński, D.; Petit, C.; Kiennemann, A. Hydrogen production from biomass gasification on nickel catalysts: Tests for dry reforming of methane. Catal. Today 2002, 76, 75–86. [Google Scholar] [CrossRef]
- Han, B.; Zhao, L.; Wang, F.; Xu, L.; Yu, H.; Cui, Y.; Zhang, J.; Shi, W. Effect of Calcination Temperature on the Performance of the Ni@SiO2 Catalyst in Methane Dry Reforming. Ind. Eng. Chem. Res. 2020, 59, 13370–13379. [Google Scholar] [CrossRef]
- Vella, L.D.; Villoria, J.A.; Specchia, S.; Mota, N.; Fierro, J.L.G.; Specchia, V. Catalytic partial oxidation of CH4 with nickel–lanthanum-based catalysts. Catal. Today 2011, 171, 84–96. [Google Scholar] [CrossRef]
- Kuras, M.; Roucou, R.; Petit, C. Studies of LaNiO3 used as a precursor for catalytic carbon nanotubes growth. J. Mol. Catal. A Chem. 2007, 265, 209–217. [Google Scholar] [CrossRef]
- Rabelo-Neto, R.C.; Sales, H.B.E.; Inocêncio, C.V.M.; Varga, E.; Oszko, A.; Erdohelyi, A.; Noronha, F.B.; Mattos, L.V. CO2 reforming of methane over supported LaNiO3 perovskite-type oxides. Appl. Catal. B Environ. 2018, 221, 349–361. [Google Scholar] [CrossRef]
- Santos, M.d.S.; Neto, R.C.R.; Noronha, F.B.; Bargiela, P.; Rocha, M.d.G.C.d.; Resini, C.; Carbó-Argibay, E.; Fréty, R.; Brandão, S.T. Perovskite as catalyst precursors in the partial oxidation of methane: The effect of cobalt, nickel and pretreatment. Catal. Today 2018, 299, 229–241. [Google Scholar] [CrossRef]
- Kuras, M.; Zimmermann, Y.; Petit, C. Reactivity of perovskite-type precursor in MWCNTs synthesis. Catal. Today 2008, 138, 55–61. [Google Scholar] [CrossRef]
- Da Silva, A.A.A.; da Costa, L.O.O.; Mattos, L.V.; Noronha, F.B. The study of the performance of Ni-based catalysts obtained from LaNiO3 perovskite-type oxides synthesized by the combustion method for the production of hydrogen by reforming of ethanol. Catal. Today 2013, 213, 25–32. [Google Scholar] [CrossRef]
- Batiot-Dupeyrat, C.; Gallego, G.A.S.; Mondragon, F.; Barrault, J.; Tatibouët, J.-M. CO2 reforming of methane over LaNiO3 as precursor material. Catal. Today 2005, 107–108, 474–480. [Google Scholar] [CrossRef]
- Sierra Gallego, G.; Batiot-Dupeyrat, C.; Barrault, J.; Mondragón, F. Dual Active-Site Mechanism for Dry Methane Reforming over Ni/La2O3 Produced from LaNiO3 Perovskite. Ind. Eng. Chem. Res. 2008, 47, 9272–9278. [Google Scholar] [CrossRef]
- He, L.; Ren, Y.; Yue, B.; Tsang, S.C.E.; He, H. Tuning Metal–Support Interactions on Ni/Al2O3 Catalysts to Improve Catalytic Activity and Stability for Dry Reforming of Methane. Processes 2021, 9, 706. [Google Scholar] [CrossRef]
- Wang, Y.-H.; Liu, H.-M.; Xu, B.-Q. Durable Ni/MgO catalysts for CO2 reforming of methane: Activity and metal–support interaction. J. Mol. Catal. A Chem. 2009, 299, 44–52. [Google Scholar] [CrossRef]
- Gallego, G.S.; Mondragón, F.; Barrault, J.; Tatibouët, J.-M.; Batiot-Dupeyrat, C. CO2 reforming of CH4 over La–Ni based perovskite precursors. Appl. Catal. A Gen. 2006, 311, 164–171. [Google Scholar] [CrossRef]
- Mosayebi, A.; Nasabi, M. Steam methane reforming on LaNiO3 perovskite-type oxide for syngas production, activity tests, and kinetic modeling. Int. J. Energy Res. 2020, 44, 5500–5515. [Google Scholar] [CrossRef]
- Lutz, A.E.; Bradshaw, R.W.; Keller, J.O.; Witmer, D.E. Thermodynamic analysis of hydrogen production by steam reforming. Int. J. Hydrog. Energy 2003, 28, 159–167. [Google Scholar] [CrossRef]
- Carapellucci, R.; Giordano, L. Steam, dry and autothermal methane reforming for hydrogen production: A thermodynamic equilibrium analysis. J. Power Sources 2020, 469, 228391. [Google Scholar] [CrossRef]
- Rostrup-Nielsen, J.R.; Sehested, J.; Nørskov, J.K. Hydrogen and Synthesis Gas by Steam and CO2 Reforming. In Advances in Catalysis; Academic Press: Cambridge, MA, USA, 2002; Volume 47, pp. 65–139. [Google Scholar]
- Rostrup-Nielsen, J.; Nørskov, J.K. Step sites in syngas catalysis. Top. Catal. 2006, 40, 45–48. [Google Scholar] [CrossRef]
- Wei, J.; Iglesia, E. Structural requirements and reaction pathways in methane activation and chemical conversion catalyzed by rhodium. J. Catal. 2004, 225, 116–127. [Google Scholar] [CrossRef]
- Hoang, D.L.; Chan, S.H.; Ding, O.L. Kinetic and modelling study of methane steam reforming over sulfide nickel catalyst on a gamma alumina support. Chem. Eng. J. 2005, 112, 1–11. [Google Scholar] [CrossRef]
- Hou, K.; Hughes, R. The kinetics of methane steam reforming over a Ni/α-Al2O catalyst. Chem. Eng. J. 2001, 82, 311–328. [Google Scholar] [CrossRef]
- Rostrup-Nielsen, J.R. Production of synthesis gas. Catal. Today 1993, 18, 305–324. [Google Scholar] [CrossRef]
- Aparicio, L.M. Transient Isotopic Studies and Microkinetic Modeling of Methane Reforming over Nickel Catalysts. J. Catal. 1997, 165, 262–274. [Google Scholar] [CrossRef]
- Mickevičius, S.; Grebinskij, S.; Bondarenka, V.; Vengalis, B.; Šliužienė, K.; Orlowski, B.A.; Osinniy, V.; Drube, W. Investigation of epitaxial LaNiO3−x thin films by high-energy XPS. J. Alloys Compd. 2006, 423, 107–111. [Google Scholar] [CrossRef]
- Serrano-Lotina, A.; Rodríguez, L.; Muñoz, G.; Martin, A.J.; Folgado, M.A.; Daza, L. Biogas reforming over La-NiMgAl catalysts derived from hydrotalcite-like structure: Influence of calcination temperature. Catal. Commun. 2011, 12, 961–967. [Google Scholar] [CrossRef]
- Abbas, S.Z.; Dupont, V.; Mahmud, T. Kinetics study and modelling of steam methane reforming process over a NiO/Al2O3 catalyst in an adiabatic packed bed reactor. Int. J. Hydrog. Energy 2017, 42, 2889–2903. [Google Scholar] [CrossRef]
- Imran, U.; Ahmad, A.; Othman, M. Kinetic Based Simulation of Methane Steam Reforming and Water Gas Shift for Hydrogen Production Using Aspen Plus. Chem. Eng. Trans. 2017, 56, 1681. [Google Scholar]
- Helveg, S.; López-Cartes, C.; Sehested, J.; Hansen, P.L.; Clausen, B.S.; Rostrup-Nielsen, J.R.; Abild-Pedersen, F.; Nørskov, J.K. Atomic-scale imaging of carbon nanofibre growth. Nature 2004, 427, 426–429. [Google Scholar] [CrossRef]
- Sierra Gallego, G.; Mondragón, F.; Tatibouët, J.-M.; Barrault, J.; Batiot-Dupeyrat, C. Carbon dioxide reforming of methane over La2NiO4 as catalyst precursor—Characterization of carbon deposition. Catal. Today 2008, 133–135, 200–209. [Google Scholar] [CrossRef]
- Hufschmidt, D.; Bobadilla, L.F.; Romero-Sarria, F.; Centeno, M.A.; Odriozola, J.A.; Montes, M.; Falabella, E. Supported nickel catalysts with a controlled molecular architecture for the catalytic reformation of methane. Catal. Today 2010, 149, 394–400. [Google Scholar] [CrossRef]
- Abasaeed, A.; Kasim, S.; Khan, W.; Sofiu, M.; Ibrahim, A.; Fakeeha, A.; Al-Fatesh, A. Hydrogen Yield from CO2 Reforming of Methane: Impact of La2O3 Doping on Supported Ni Catalysts. Energies 2021, 14, 2412. [Google Scholar] [CrossRef]
- Liu, B.S.; Au, C.T. Carbon deposition and catalyst stability over La2NiO4/γ-Al2O3 during CO2 reforming of methane to syngas. Appl. Catal. A Gen. 2003, 244, 181–195. [Google Scholar] [CrossRef]
- Habibi, N.; Rezaei, M.; Majidian, N.; Andache, M. CH4 reforming with CO2 for syngas production over La2O3 promoted Ni catalysts supported on mesoporous nanostructured lambda-Al2O3. J. Energy Chem. 2014, 23, 435–442. [Google Scholar] [CrossRef]
- Bicki, R.; Antoniak-Jurak, K.; Michalska, K.; Franczyk, E.; Konkol, M.; Kowalik, P.; Panczyk, M.; Ryczkowski, J.; Slowik, G.; Borowiecki, T. The effect of La2O3 and CeO2 modifiers on properties of Ni-Al catalysts for LNG prereforming. Int. J. Hydrog. Energy 2021, 46, 11664–11676. [Google Scholar] [CrossRef]
- Tuza, P.V.; Souza, M.M.V.M. Steam Reforming of Methane Over Catalyst Derived from Ordered Double Perovskite: Effect of Crystalline Phase Transformation. Catal. Lett. 2015, 146, 47–53. [Google Scholar] [CrossRef]
- Provendier, H.; Petit, C.; Roger, A.C.; Kiennemann, A. Influence of the Precursors on the Formation of a Trimetallic Defined Structure. Application on Ni Catalysts Used for Syngas Obtention. Stud. Surf. Sci. Catal. 1998, 118, 285–294. [Google Scholar]
- De Lima, S.M.; da Silva, A.M.; da Costa, L.O.O.; Assaf, J.M.; Jacobs, G.; Davis, B.H.; Mattos, L.V.; Noronha, F.B. Evaluation of the performance of Ni/La2O3 catalyst prepared from LaNiO3 perovskite-type oxides for the production of hydrogen through steam reforming and oxidative steam reforming of ethanol. Appl. Catal. A Gen. 2010, 377, 181–190. [Google Scholar] [CrossRef]
















| T (°C) | Weight Loss (%) | Gas Products | Heat Effect | Thermal Events | |
|---|---|---|---|---|---|
| Step 1 | 30–140 | 2 | H2O | − | Dehydration |
| Step 2 | 140–360 | 38 | CO2, H2O, NO | − | Decomposition of nitrate; oxidation of organic groups |
| Step 3 | 360–570 | 28 | CO2, H2O | + | Oxidation of organic components; LaNiO3 crystallization |
| Step 4 | 570–700 | 2 | CO2 | − | Decomposition of lanthanum oxycarbonate; LaNiO3 crystallization |
| Step 5 | 700–900 | 0 | None | − | LaNiO3 crystallization |
| Sample | SSABET/m2·g−1 | D(LaNiO3)/nm | Distorted Rhombohedral Symmetry | D(Ni0)/nm | T (Reduction Peak)/°C | ||
|---|---|---|---|---|---|---|---|
| a/Å | c/Å | V/Å3 | |||||
| LNO-C600 | 10.796 | 10.0 | 5.474 | 6.738 | 169.84 | 30.0 | 305; 349; 514 |
| LNO-C700 | 8.033 | 13.9 | 5.453 | 6.636 | 169.20 | 26.5 | 370; 526 |
| LNO-C800 | 4.579 | 15.8 | 5.440 | 6.585 | 168.79 | 22.8 | 387; 536 |
| LNO-C900 | 2.544 | 17.9 | 5.430 | 5.579 | 168.15 | 19.8 | 387; 545 |
| Catalysts | mg C/mg Catalyst (%) | T (Carbon Elimination Peak)/°C |
|---|---|---|
| LNO-C600 | 18.56 | 610 |
| LNO-C700 | 9.85 | 580 |
| LNO-C800 | 0.56 | 498 |
| LNO-C900 | 0.50 | 497 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Qian, S.; Chen, Y.; Yan, B.; Cheng, Y. Effect of the Calcination Temperature of LaNiO3 on the Structural Properties and Reaction Performance of Catalysts in the Steam Reforming of Methane. Catalysts 2023, 13, 356. https://doi.org/10.3390/catal13020356
Wang Y, Qian S, Chen Y, Yan B, Cheng Y. Effect of the Calcination Temperature of LaNiO3 on the Structural Properties and Reaction Performance of Catalysts in the Steam Reforming of Methane. Catalysts. 2023; 13(2):356. https://doi.org/10.3390/catal13020356
Chicago/Turabian StyleWang, Yujie, Shuairen Qian, Yuxin Chen, Binhang Yan, and Yi Cheng. 2023. "Effect of the Calcination Temperature of LaNiO3 on the Structural Properties and Reaction Performance of Catalysts in the Steam Reforming of Methane" Catalysts 13, no. 2: 356. https://doi.org/10.3390/catal13020356