Quantification of the Microwave Effect in the Synthesis of 5-Hydroxymethylfurfural over Sulfonated MIL-101(Cr)
Abstract
:1. Introduction
2. Results and Discussion
2.1. Preliminary Screening of the Dehydration Conditions
2.1.1. Sulfonic Acid Group Loading Effect
2.1.2. Solvent Effect on Fructose Dehydration Using MW Heating
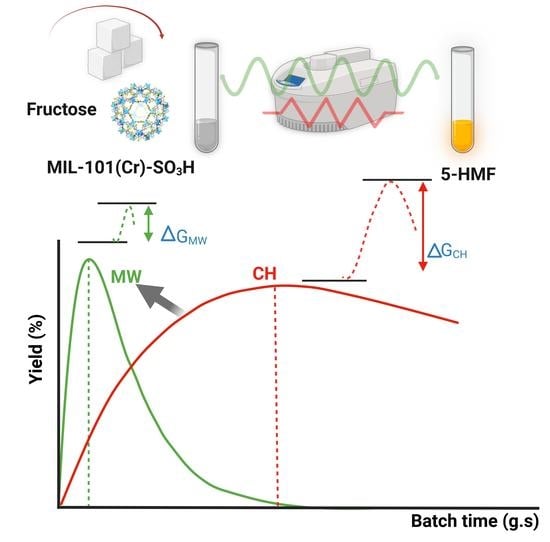
2.1.3. Batch Time Evolution
2.2. Multiresponse Kinetic Modeling and Reaction Conditions Optimization
2.2.1. Pre-Exponential Factors and Activation Energies under CH Conditions
2.2.2. Quantification of the Microwave Irradiation Effect
2.2.3. Maximum 5-HMF Yield as a Function of Temperature
3. Materials and Methods
3.1. Chemicals and Catalysts
3.2. Catalyst Testing
3.2.1. General Procedure for the Reaction of Fructose Using Microwave Irradiation
3.2.2. General Procedure for the Dehydration Reaction of Fructose Using Conventional Heating
3.3. Analysis
3.4. Procedure for Multiresponse Kinetic Modeling
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations and Acronyms
| A | pre-exponential factor | min−1 |
| Ac | acetone | |
| avg | average | |
| b | bulk | |
| cat | catalyst | |
| CH | conventional heating | |
| DMSO | dimethyl sulfoxide | |
| Ei,a | activation energy | kJ·mol−1 |
| EL | ethyl levulinate | |
| EMF | 5-ethoxymethylfurfural | |
| FA | formic acid | |
| FDCA | 2,5-furandicarboxylic acid | |
| fru | fructose | |
| GVL | γ-valerolactone | |
| H | enthalpy | kJ·mol−1 |
| HMF | hydroxymethylfurfural | |
| HPD | highest posterior density | |
| HPLC | high-performance liquid chromatography | |
| Int. | intermediate | |
| k | reaction rate constant for fructose dehydration | Mmol·min−1 |
| LA | levulinic acid | |
| MIL | material of the Institute Lavoisier | |
| MW | microwave | |
| R | universal gas constant | J·mol−1 K−1 |
| RID | refractive index detector | |
| SPCs | secondary building units | |
| S | entropy | J·mol−1 K−1 |
| sub | substrate | |
| t | time | min |
| T | temperature | K |
| x | conversion | mol·mol−1 |
| δ | tangent delta | |
| ε | dielectric constant | |
| ε″ | dielectric loss |
References
- Aljammal, N.; Jabbour, C.; Thybaut, J.W.; Demeestere, K.; Verpoort, F.; Heynderickx, P.M. Metal-Organic Frameworks as Catalysts for Sugar Conversion into Platform Chemicals: State-of-the-Art and Prospects. Coord. Chem. Rev. 2019, 401, 213064. [Google Scholar] [CrossRef]
- Heidenreich, S.; Müller, M.; Foscolo, P.U. Biomass Pretreatment. In Advanced Biomass Gasification; New Concepts for Efficiency Increase and Product Flexibility; Elsevier: Amsterdam, The Netherlands, 2016; pp. 11–17. ISBN 9780128042960. [Google Scholar]
- Bozell, J.J.; Petersen, G.R. Technology Development for the Production of Biobased Products from Biorefinery Carbohydrates—The US Department of Energy’s “Top 10” Revisited. Green Chem. 2010, 12, 539–555. [Google Scholar] [CrossRef]
- Kuster, B.F.M. 5-Hydroxymethylfurfural (HMF). A Review Focussing on Its Manufacture. Starch-Stärke 1990, 42, 314–321. [Google Scholar] [CrossRef] [Green Version]
- Lewkowski, J. Synthesis, Chemistry and Applications of 5-Hydroxymethyl-Furfural and Its Derivatives. Arkivoc 2001, 2001, 17–54. [Google Scholar] [CrossRef] [Green Version]
- Tahvildari, K.; Taghvaei, S.; Nozari, M. The Study of Hydroxymethylfurfural as a Basic Reagent for Liquid Alkanes Fuel Manufacture from Agricultural Wastes. Int. J. Chem. Environ. Eng. 2011, 2, 62–68. [Google Scholar]
- Fulignati, S.; Antonetti, C.; Tabanelli, T.; Cavani, F.; Raspolli Galletti, A.M. Integrated Cascade Process for the Catalytic Conversion of 5-Hydroxymethylfurfural to Furanic and TetrahydrofuranicDiethers as Potential Biofuels. ChemSusChem 2022, 15, e202200241. [Google Scholar] [CrossRef]
- Tong, X.; Ma, Y.; Li, Y. Biomass into Chemicals: Conversion of Sugars to Furan Derivatives by Catalytic Processes. Appl. Catal. A Gen. 2010, 385, 1–13. [Google Scholar] [CrossRef]
- Noppadon, S.; Allendorf, M.D.; George, A.; Jansen, R.; Leong, K.; Simmons, B.A.; Singh, S.; Travisano, P. Metal Organic Frameworks for the Conversion of Lignocellulosic Derivatives to Renewable Platform Chemicals 2019. US20190062293A1, 28 February 2019. [Google Scholar]
- Nainamalai Devarajan and Palaniswamy Suresh MIL-101-SO3H Metal-Organic Framework as a Brønsted Acid Catalyst in Hantzsch Reaction: An Efficient and Sustainable Methodology for One-Pot Synthesis of 1,4-Dihydropyridine. New J. Chem. 2019, 43, 6806–6814. [CrossRef]
- Guan, W.; Zhang, Y.; Wei, Y.; Li, B.; Feng, Y.; Yan, C.; Huo, P.; Yan, Y. Pickering HIPEs Derived Hierarchical Porous Nitrogen-Doped Carbon Supported Bimetallic AuPd Catalyst for Base-Free Aerobic Oxidation of HMF to FDCA in Water. Fuel 2020, 278, 118362. [Google Scholar] [CrossRef]
- Martínez-Vargas, D.X.; Rivera De La Rosa, J.; Sandoval-Rangel, L.; Guzmán-Mar, J.L.; Garza-Navarro, M.A.; Lucio-Ortiz, C.J.; De Haro-Del Río, D.A. 5-Hydroxymethylfurfural Catalytic Oxidation under Mild Conditions by Co (II), Fe (III) and Cu (II) Salen Complexes Supported on SBA-15: Synthesis, Characterization and Activity. Appl. Catal. A Gen. 2017, 547, 132–145. [Google Scholar] [CrossRef]
- Luo, W.; Sankar, M.; Beale, A.M.; He, Q.; Kiely, C.J.; Bruijnincx, P.C.A.; Weckhuysen, B.M. High Performing and Stable Supported Nano-Alloys for the Catalytic Hydrogenation of Levulinic Acid to γ-Valerolactone. Nat. Commun. 2015, 6, 6540. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Zhao, G.; Chen, L. Efficient Production of 5-Hydroxymethylfurfural and Alkyl Levulinate from Biomass Carbohydrate Using Ionic Liquid-Based Polyoxometalate Salts. RSC Adv. 2014, 4, 4194–4202. [Google Scholar] [CrossRef]
- Liu, A.; Zhang, Z.; Fang, Z.; Liu, B.; Huang, K. Synthesis of 5-Ethoxymethylfurfural from 5-Hydroxymethylfurfural and Fructose in Ethanol Catalyzed by MCM-41 Supported Phosphotungstic Acid. J. Ind. Eng. Chem. 2014, 20, 1977–1984. [Google Scholar] [CrossRef]
- Ouyang, W.; Zhao, D.; Wang, Y.; Balu, A.M.; Len, C.; Luque, R. Continuous Flow Conversion of Biomass-Derived Methyl Levulinate into γ-Valerolactone Using Functional Metal Organic Frameworks. ACS Sustain. Chem. Eng. 2018, 6, 6746–6752. [Google Scholar] [CrossRef]
- Horváth, I.T.; Mehdi, H.; Fábos, V.; Boda, L.; Mika, L.T. γ-Valerolactone—A Sustainable Liquid for Energy and Carbon-Based Chemicals. Green Chem. 2008, 10, 238–242. [Google Scholar] [CrossRef]
- Huber, G.W.; Corma, A. Synergies between Bio- and Oil Refineries for the Production of Fuels from Biomass. Angew. Chem. Int. Ed. 2007, 46, 7184–7201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Insyani, R.; Verma, D.; Kim, S.M.; Kim, J. Direct One-Pot Conversion of Monosaccharides into High-Yield 2,5-Dimethylfuran over a Multifunctional Pd/Zr-Based Metal-Organic Framework@sulfonated Graphene Oxide Catalyst. Green Chem. 2017, 19, 2482–2490. [Google Scholar] [CrossRef]
- Fang, R.; Luque, R.; Li, Y. Efficient One-Pot Fructose to DFF Conversion Using Sulfonated Magnetically Separable MOF-Derived Fe3O4(111) Catalysts. Green Chem. 2017, 19, 647–655. [Google Scholar] [CrossRef]
- Fang, R.; Luque, R.; Li, Y. Selective Aerobic Oxidation of Biomass-Derived HMF to 2,5-Diformylfuran Using a MOF-Derived Magnetic Hollow Fe-Co Nanocatalyst. Green Chem. 2016, 18, 3152–3157. [Google Scholar] [CrossRef]
- Liu, R.; Chen, J.; Chen, L.; Guo, Y.; Zhong, J. One-Step Approach to 2,5-Diformylfuran from Fructose by Using a Bifunctional and Recyclable Acidic Polyoxometalate Catalyst. Chempluschem 2014, 79, 1448–1454. [Google Scholar] [CrossRef]
- Tao, F.; Cui, Y.; Yang, P.; Gong, Y. One-Pot, One-Step, Catalytic Synthesis of 2,5-Diformylfuran from Fructose. Russ. J. Phys. Chem. A 2014, 88, 1091–1096. [Google Scholar] [CrossRef]
- Zhang, F.; Liu, Y.; Yuan, F.; Niu, X.; Zhu, Y. Efficient Production of the Liquid Fuel 2,5-Dimethylfuran from 5-Hydroxymethylfurfural in the Absence of Acid Additive over Bimetallic PdAu Supported on Graphitized Carbon. Energy Fuels 2017, 31, 6364–6373. [Google Scholar] [CrossRef]
- Choudhary, V.; Mushrif, S.H.; Ho, C.; Anderko, A.; Nikolakis, V.; Marinkovic, N.S.; Frenkel, A.I.; Sandler, S.I.; Vlachos, D.G. Insights into the Interplay of Lewis and Brønsted Acid Catalysts in Glucose and Fructose Conversion to 5-(Hydroxymethyl)Furfural and Levulinic Acid in Aqueous Media. J. Am. Chem. Soc. 2013, 135, 3997–4006. [Google Scholar] [CrossRef]
- Yang, F.; Liu, Q.; Bai, X.; Du, Y. Conversion of Biomass into 5-Hydroxymethylfurfural Using Solid Acid Catalyst. Bioresour. Technol. 2011, 102, 3424–3429. [Google Scholar] [CrossRef]
- Tao, F.; Song, H.; Chou, L. Dehydration of Fructose into 5-Hydroxymethylfurfural in Acidic Ionic Liquids. RSC Adv. 2011, 1, 672–676. [Google Scholar] [CrossRef]
- Steinbach, D.; Kruse, A.; Sauer, J.; Vetter, P. Sucrose Is a Promising Feedstock for the Synthesis of the Platform Chemical Hydroxymethylfurfural. Energies 2018, 11, 645. [Google Scholar] [CrossRef] [Green Version]
- Zhou, C.; Zhao, J.; Yagoub, A.E.G.A.; Ma, H.; Yu, X.; Hu, J.; Bao, X.; Liu, S. Conversion of Glucose into 5-Hydroxymethylfurfural in Different Solvents and Catalysts: Reaction Kinetics and Mechanism. Egypt. J. Pet. 2017, 26, 477–487. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Zhuo, Y.; Shanks, K.; Taylor, R.A.; Conneely, B.; Tan, A.; Shen, Y.; Scott, J. A Winged Solar Biomass Reactor for Producing 5-Hydroxymethylfurfural (5-HMF). Sol. Energy 2021, 218, 455–468. [Google Scholar] [CrossRef]
- Jia, S.; He, X.; Xu, Z. Valorization of an Underused Sugar Derived from Hemicellulose: Efficient Synthesis of 5-Hydroxymethylfurfural from Mannose with Aluminum Salt Catalyst in Dimethyl Sulfoxide/Water Mixed Solvent. RSC Adv. 2017, 7, 39221–39227. [Google Scholar] [CrossRef] [Green Version]
- Menegazzo, F.; Ghedini, E.; Signoretto, M. 5-Hydroxymethylfurfural (HMF) Production from Real Biomasses. Molecules 2018, 23, 2201. [Google Scholar] [CrossRef] [Green Version]
- Sansuk, S.; Subsadsana, M. Synthesis of 5-Hydroxymethylfurfural from Glucose Using H-Beta Catalyst Treated with Phosphoric Acid in One-Pot Biphasic Solvent System. Energy Sources Part A Recover. Util. Environ. Eff. 2019, 41, 2769–2777. [Google Scholar] [CrossRef]
- Zhang, Y.; Pidko, E.A.; Hensen, E.J.M. Molecular Aspects of Glucose Dehydration by Chromium Chlorides in Ionic Liquids. Chem. A Eur. J. 2011, 17, 5281–5288. [Google Scholar] [CrossRef]
- Sheldon, R.A. Green Solvents for Sustainable Organic Synthesis: State of the Art. Green Chem. 2005, 7, 267–278. [Google Scholar] [CrossRef]
- Richel, A.; Paquot, M. Conversion of Carbohydrates under Microwave Heating. In Carbohydrates-Comprehensive Studies on Glycobiology and Glycotechnology; IntechOpen: London, UK, 2012. [Google Scholar]
- Hu, D.; Zhang, M.; Xu, H.; Wang, Y.; Yan, K. Recent Advance on the Catalytic System for Efficient Production of Biomass-Derived 5-Hydroxymethylfurfural. Renew. Sustain. Energy Rev. 2021, 147, 111253. [Google Scholar] [CrossRef]
- Flores, E.M.M.; Cravotto, G.; Bizzi, C.A.; Santos, D.; Iop, G.D. Ultrasound-Assisted Biomass Valorization to Industrial Interesting Products: State-of-the-Art, Perspectives and Challenges. Ultrason. Sonochem. 2021, 72, 105455. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Smith, R.L.; Qi, X. Production of Biofuels and Chemicals with Microwave; Springer: Berlin/Heidelberg, Germany, 2015; Volume 3, ISBN 978-981-10-5136-4. [Google Scholar]
- Siddique, I.J.; Salema, A.A.; Antunes, E.; Vinu, R. Recent Advance on the Catalytic System for Efficient Production of Biomass-Derived 5-Hydroxymethylfurfural. Renew. Sustain. Energy Rev. 2022, 153, 111767. [Google Scholar] [CrossRef]
- Gude, V.G.; Patil, P.; Deng, S. Recent Advance on the Catalytic System for Efficient Production of Biomass-Derived 5-Hydroxymethylfurfural. In Proceedings of the World Renewable Energy Forum, WREF 2012, Including World Renewable Energy Congress XII and Colorado Renewable Energy Society (CRES) Annual Conference, Denver, CO, USA, 13–17 May 2012; Volume 1, pp. 751–759. [Google Scholar]
- Leadbeater, N.E. Microwave Heating as a Tool for Sustainable Chemistry; CRC Press: Boca Raton, FL, USA, 2010; ISBN 9781439812709. [Google Scholar]
- Gillespie, P.M. Microwave Chemistry—An Approach to the Assessment of Chemical Reaction Hazards. IChemE Symp. Ser. No 2004, 10, 23–25. [Google Scholar]
- Rosana, M.R.; Hunt, J.; Ferrari, A.; Southworth, T.A.; Tao, Y.; Stiegman, A.E.; Dudley, G.B. Microwave-Specific Acceleration of a Friedel-Crafts Reaction: Evidence for Selective Heating in Homogeneous Solution. J. Org. Chem. 2014, 79, 7437–7450. [Google Scholar] [CrossRef]
- Guzik, P.; Kulawik, P.; Zając, M.; Migdał, W. Microwave Applications in the Food Industry: An Overview of Recent Developments. Crit. Rev. Food Sci. Nutr. 2021, 62, 7989–8008. [Google Scholar] [CrossRef]
- Tabasso, S. Microwave-Assisted Biomass Conversion. In Microwave Chemistry; De Gruyter: Berlin, Germany, 2017; pp. 370–382. ISBN 9783110479935. [Google Scholar]
- Kim, E.S.; Liu, S.; Abu-Omar, M.M.; Mosier, N.S. Selective Conversion of Biomass Hemicellulose to Furfural Using Maleic Acid with Microwave Heating. Energy Fuels 2012, 26, 1298–1304. [Google Scholar] [CrossRef]
- Sweygers, N. The Microwave-Assisted Production of 5-Hydroxymethylfurfural and Furfural from Renewable Resources; KU Leuven: Leuven, Belgium, 2019. [Google Scholar]
- Hansen, T.S.; Woodley, J.M.; Riisager, A. Efficient Microwave-Assisted Synthesis of 5-Hydroxymethylfurfural from Concentrated Aqueous Fructose. Carbohydr. Res. 2009, 344, 2568–2572. [Google Scholar] [CrossRef] [PubMed]
- Wei, R.; Wang, P.; Zhang, G.; Wang, N.; Zheng, T. Microwave-Responsive Catalysts for Wastewater Treatment: A Review. Chem. Eng. J. 2020, 382, 122781. [Google Scholar] [CrossRef]
- Qin, M.; Zhang, L.; Wu, H. Dielectric Loss Mechanism in Electromagnetic Wave Absorbing Materials. Adv. Sci. 2022, 9, 2105553. [Google Scholar] [CrossRef]
- Martín, Á.; Navarrete, A. Microwave-Assisted Process Intensification Techniques. Curr. Opin. Green Sustain. Chem. 2018, 11, 70–75. [Google Scholar] [CrossRef]
- Hayes, B.L. Microwave Synthesis, Chemistry at the Speed of Light; CEM Corp: Charlotte, NC, USA, 2002. [Google Scholar]
- Polshettiwar, V.; Varma, R.S. Microwave-Assisted Organic Synthesis and Transformations Using Benign Reaction Media. Acc. Chem. Res. 2008, 41, 629–639. [Google Scholar] [CrossRef]
- Herrero, M.A.; Kremsner, J.M.; Kappe, C.O.; Graz, K.V.; Graz, A. Nonthermal Microwave Effects Revisited: On the Importance of Internal Temperature Monitoring and Agitation in Microwave Chemistry. J. Org. Chem. 2008, 73, 36–47. [Google Scholar] [CrossRef]
- Kappe, C.O.; Pieber, B.; Dallinger, D. Microwave Effects in Organic Synthesis: Myth or Reality? Angew. Chem. Int. Ed. 2013, 52, 1088–1094. [Google Scholar] [CrossRef]
- Kappe, C.O. Reply to the Correspondence on Microwave Effects in Organic Synthesis. Angew. Chem. Int. Ed. 2013, 52, 2–7. [Google Scholar] [CrossRef]
- Dudley, G.B.; Stiegman, A.E.; Rosana, M.R. Correspondence on Microwave Effects in Organic Synthesis. Angew. Chem. Int. Ed. 2013, 52, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Rosana, M.R.; Dudley, G.B.; Stiegman, A.E. Parameters Affecting the Microwave-Specifi Acceleration of a Chemical Reaction. JOC 2014, 79, 7425–7436. [Google Scholar] [CrossRef]
- Hunt, J.T. Microwave Enhanced Gasification of Carbon; Florida State University: Tallahassee, FL, USA, 2014. [Google Scholar]
- Fini, A.; Breccia, A. Chemistry by Microwaves. Pure Appl. Chem. 1999, 71, 573–579. [Google Scholar] [CrossRef]
- Perreux, L.; Loupy, A.; Alain, P. Nonthermal Effects of Microwaves in Organic Synthesis. In Microwaves in Organic Synthesis; John Wiley & Sons: Hoboken, NJ, USA, 2013; pp. 127–207. [Google Scholar]
- Miklavc, A. Strong Acceleration of Chemical Reactions Occurring through the Effects of Rotational Excitation on Collision Geometry. ChemPhysChem 2001, 2, 552–555. [Google Scholar] [CrossRef]
- Hu, J.; Wildfire, C.; Stiegman, A.E.; Dagle, R.A.; Shekhawat, D.; Abdelsayed, V.; Bai, X.; Tian, H.; Bogle, M.B.; Hsu, C.; et al. Microwave-Driven Heterogeneous Catalysis for Activation of Dinitrogen to Ammonia under Atmospheric Pressure. Chem. Eng. J. 2020, 397, 125388. [Google Scholar] [CrossRef]
- Horikoshi, S.; Minagawa, T.; Tsubaki, S.; Onda, A.; Serpone, N. Is Selective Heating of the Sulfonic Acid Catalyst AC-SO3H by Microwave Radiation Crucial in the Acid Hydrolysis of Cellulose to Glucose in Aqueous Media? Catalysts 2017, 7, 231. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Hayward, D.O.; Mingos, D.M.P. Apparent Equilibrium Shifts and Hot-Spot Formation for Catalytic Reactions Induced by Microwave Dielectric Heating. Chem. Commun. 1999, 11, 975–976. [Google Scholar] [CrossRef] [Green Version]
- Kokel, A.; Schäfer, C.; Török, B. Application of Microwave-Assisted Heterogeneous Catalysis in Sustainable Synthesis Design. Green Chem. 2017, 19, 3729–3751. [Google Scholar] [CrossRef]
- Herbst, A.; Janiak, C. Selective Glucose Conversion to 5-Hydroxymethylfurfural (5-HMF) Instead of Levulinic Acid with MIL-101Cr MOF-Derivatives. New J. Chem. 2016, 40, 7958–7967. [Google Scholar] [CrossRef] [Green Version]
- Herbst, A.; Janiak, C. MOF Catalysts in Biomass Upgrading towards Value-Added Fine Chemicals. CrystEngComm 2017, 19, 4092–4117. [Google Scholar] [CrossRef] [Green Version]
- Oozeerally, R.; Ramkhelawan, S.D.K.; Burnett, D.L.; Tempelman, C.H.L.; Degirmenci, V. ZIF-8 Metal Organic Framework for the Conversion of Glucose to Fructose and 5-Hydroxymethyl Furfural. Catalysts 2019, 9, 812. [Google Scholar] [CrossRef] [Green Version]
- Oozeerally, R.; Burnett, D.L.; Chamberlain, T.W.; Walton, R.I.; Degirmenci, V. Exceptionally Efficient and Recyclable Heterogeneous Metal–Organic Framework Catalyst for Glucose Isomerization in Water. ChemCatChem 2018, 10, 706–709. [Google Scholar] [CrossRef]
- Pertiwi, R.; Oozeerally, R.; Burnett, D.L.; Chamberlain, T.W.; Cherkasov, N.; Walker, M.; Kashtiban, R.J.; Krisnandi, Y.K.; Degirmenci, V.; Walton, R.I. Replacement of Chromium by Non-Toxic Metals in Lewis-Acid MOFs: Assessment of Stability as Glucose Conversion Catalysts. Catalysts 2019, 9, 437. [Google Scholar] [CrossRef] [Green Version]
- Deng, H.; Grunder, S.; Cordova, K.E.; Valente, C.; Furukawa, H.; Hmadeh, M.; Gándara, F.; Whalley, A.C.; Liu, Z.; Asahina, S.; et al. Large-Pore Apertures in a Series of Metal-Organic Frameworks. Science 2012, 336, 1018–1023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tranchemontagne, D.J.; Ni, Z.; O’Keeffe, M.; Yaghi, O.M. Reticular Chemistry of Metal-Organic Polyhedra. Angew. Chem. Int. Ed. 2008, 47, 5136–5147. [Google Scholar] [CrossRef]
- Kalmutzki, M.J.; Diercks, C.S.; Yaghi, O.M. Metal–Organic Frameworks for Water Harvesting from Air. Adv. Mater. 2018, 30, e1704304. [Google Scholar] [CrossRef] [PubMed]
- Britt, D.; Tranchemontagne, D.; Yaghi, O.M. Metal-Organic Frameworks with High Capacity and Selectivity for Harmful Gases. Proc. Natl. Acad. Sci. USA 2008, 105, 11623–11627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eddaoudi, M.; Kim, J.; Rosi, N.; Vodak, D.; Wachter, J.; O’Keeffe, M.; Yaghi, O.M. Systematic Design of Pore Size and Functionality in Isoreticular MOFs and Their Application in Methane Storage. Science 2002, 295, 469–472. [Google Scholar] [CrossRef] [Green Version]
- Aljammal, N.; Jabbour, C.; Chaemchuen, S.; Juzsakova, T.; Verpoort, F. Flexibility in Metal–Organic Frameworks: A Basic Understanding. Catalysts 2019, 9, 512. [Google Scholar] [CrossRef] [Green Version]
- Luo, Z.; Chaemchuen, S.; Zhou, K.; Verpoort, F. Ring-Opening Polymerization of l-Lactide to Cyclic Poly(Lactide) by Zeolitic Imidazole Framework ZIF-8 Catalyst. ChemSusChem 2017, 10, 4135–4139. [Google Scholar] [CrossRef]
- Chughtai, A.H.; Ahmad, N.; Younus, H.A.; Laypkov, A.; Verpoort, F. Metal-Organic Frameworks: Versatile Heterogeneous Catalysts for Efficient Catalytic Organic Transformations. Chem. Soc. Rev. 2015, 44, 6804–6849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yabushita, M.; Li, P.; Islamoglu, T.; Kobayashi, H.; Fukuoka, A.; Farha, O.K.; Katz, A. Selective Metal-Organic Framework Catalysis of Glucose to 5-Hydroxymethylfurfural Using Phosphate-Modified NU-1000. Ind. Eng. Chem. Res. 2017, 56, 7141–7148. [Google Scholar] [CrossRef]
- Aljammal, N.; Lenssens, A.; Reviere, A.; Verberckmoes, A.; Thybaut, J.W.; Verpoort, F.; Heynderickx, P.M. Metal–Organic Frameworks as Catalysts for Fructose Conversion into 5-hydroxymethylfurfural: Catalyst Screening and Parametric Study. Appl. Organomet. Chem. 2021, 35, e6419. [Google Scholar] [CrossRef]
- Wang, K.; Liu, Y.; Wu, W.; Chen, Y.; Fang, L.; Li, W.; Ji, H. Production of Levulinic Acid via Cellulose Conversion over Metal Oxide-Loaded MOF Catalysts in Aqueous Medium. Catal. Lett. 2020, 150, 322–331. [Google Scholar] [CrossRef]
- Chen, J.; Li, K.; Chen, L.; Liu, R.; Huang, X.; Ye, D. Conversion of Fructose into 5-Hydroxymethylfurfural Catalyzed by Recyclable Sulfonic Acid-Functionalized Metal-Organic Frameworks. Green Chem. 2014, 16, 2490–2499. [Google Scholar] [CrossRef]
- Akiyama, G.; Matsuda, R.; Sato, H.; Takata, M.; Kitagawa, S. Cellulose Hydrolysis by a New Porous Coordination Polymer Decorated with Sulfonic Acid Functional Groups. Adv. Mater. 2011, 23, 3294–3297. [Google Scholar] [CrossRef]
- Liu, S.; Meng, Y.; Li, H.; Yang, S. Hierarchical Porous Mil-101(Cr) Solid Acid-Catalyzed Production of Value-Added Acetals from Biomass-Derived Furfural. Polymers 2021, 13, 3498. [Google Scholar] [CrossRef]
- Chatterjee, A.; Hu, X.; Lam, F.L.-Y. A Dual Acidic Hydrothermally Stable MOF-Composite for Upgrading Xylose to Furfural. Appl. Catal. A Gen. 2018, 566, 130–139. [Google Scholar] [CrossRef]
- Liu, X.F.; Li, H.; Zhang, H.; Pan, H.; Huang, S.; Yang, K.L.; Yang, S. Efficient Conversion of Furfuryl Alcohol to Ethyl Levulinate with Sulfonic Acid-Functionalized MIL-101(Cr). RSC Adv. 2016, 6, 90232–90238. [Google Scholar] [CrossRef]
- Xu, S.; Pan, D.; Wu, Y.; Song, X.; Gao, L.; Li, W.; Das, L.; Xiao, G. Efficient Production of Furfural from Xylose and Wheat Straw by Bifunctional Chromium Phosphate Catalyst in Biphasic Systems. Fuel Process. Technol. 2018, 175, 90–96. [Google Scholar] [CrossRef]
- Zhao, H.; Holladay, J.E.; Brown, H.; Zhang, Z.C. Metal Chlorides in Ionic Liquid Solvents Convert Sugars to 5-Hydroxymethylfurfural. Science 2007, 316, 1597–1600. [Google Scholar] [CrossRef]
- Shao, Y.; Ding, Y.; Dai, J.; Long, Y.; Hu, Z.T. Synthesis of 5-Hydroxymethylfurfural from Dehydration of Biomass-Derived Glucose and Fructose Using Supported Metal Catalysts. Green Synth. Catal. 2021, 2, 187–197. [Google Scholar] [CrossRef]
- Paul, G.; Iga, G.D.; Cabral, N.M.; Bueno, C.; Bisio, C.; Gallo, J.M.R. General Niobium Phosphates as Bifunctional Catalysts for the Conversion of Biomass-Derived Monosaccharides. Appl. Catal. A 2021, 617, 118099. [Google Scholar] [CrossRef]
- Zhang, T.; Li, W.; Xin, H.; Jin, L.; Liu, Q. Production of HMF from Glucose Using an Al3+ -Promoted Acidic Phenol- Formaldehyde Resin Catalyst. Catal. Commun. 2019, 124, 56–61. [Google Scholar] [CrossRef]
- Palma, V.; Barba, D.; Cortese, M.; Martino, M.; Renda, S.; Meloni, E. Microwaves and Heterogeneous Catalysis: A Review on Selected Catalytic Processes. Catalysts 2020, 10, 246. [Google Scholar] [CrossRef] [Green Version]
- Bromberg, L.; Su, X.; Hatton, T.A. Functional Networks of Organic and Coordination Polymers: Catalysis of Fructose Conversion. Chem. Mater. 2014, 26, 6257–6264. [Google Scholar] [CrossRef]
- Guo, W.; Zhang, Z.; Hacking, J.; Heeres, H.J.; Yue, J. Selective Fructose Dehydration to 5-Hydroxymethylfurfural from a Fructose-Glucose Mixture over a Sulfuric Acid Catalyst in a Biphasic System: Experimental Study and Kinetic Modelling. Chem. Eng. J. 2021, 409, 128182. [Google Scholar] [CrossRef]
- Testa, M.L.; Miroddi, G.; Russo, M.; La Parola, V.; Marcì, G. Dehydration of Fructose to 5-HMF over Acidic TiO2 Catalysts. Materials 2020, 13, 1178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carraher, J.M.; Fleitman, C.N.; Tessonnier, J.P. Kinetic and Mechanistic Study of Glucose Isomerization Using Homogeneous Organic Brønsted Base Catalysts in Water. ACS Catal. 2015, 5, 3162–3173. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Wang, Y.; Xie, X.; Huang, C.; Yang, S. Dehydration of Fructose, Sucrose and Inulin to 5-Hydroxymethylfurfural over Yeast-Derived Carbonaceous Microspheres at Low Temperatures. RSC Adv. 2019, 9, 9041–9048. [Google Scholar] [CrossRef] [Green Version]
- Van Putten, R.J.; Van Der Waal, J.C.; De Jong, E.; Rasrendra, C.B.; Heeres, H.J.; De Vries, J.G. Hydroxymethylfurfural, a Versatile Platform Chemical Made from Renewable Resources. Chem. Rev. 2013, 113, 1499–1597. [Google Scholar] [CrossRef] [PubMed]
- Ji, T.; Li, Z.; Liu, C.; Lu, X.; Li, L.; Zhu, J. Niobium-Doped TiO2 Solid Acid Catalysts: Strengthened Interfacial Polarization, Amplified Microwave Heating and Enhanced Energy Efficiency of Hydroxymethylfurfural Production. Appl. Catal. B Environ. 2019, 243, 741–749. [Google Scholar] [CrossRef]
- Zhang, A.; Li, M.; Wang, D.; Li, Y.; Zhang, Q.; Kong, J. Enhanced Electromagnetic Wave Absorption of Polar Absorber Hybrids Self-Assembled by MWCNTs and Sulfonated Polystyrene Microsphere. J. Mater. Sci. 2020, 55, 1637–1647. [Google Scholar] [CrossRef]
- Li, C.; Zhang, Y.; Ji, S.; Jiang, X.; Zhang, Z.; Yu, L. Microwave Absorption Properties of γ-Fe2O3/(SiO2)x– SO3H/Polypyrrole Core/Shell/Shell Microspheres. J. Mater. Sci. 2018, 53, 5270–5286. [Google Scholar] [CrossRef]
- Nüchter, M.; Ondruschka, B.; Jungnickel, A.; Müller, U. Organic Processes Initiated by Non-Classical Energy Sources. J. Phys. Org. Chem. 2000, 13, 579–586. [Google Scholar] [CrossRef]
- Asakuma, Y.; Ogawa, Y.; Maeda, K.; Fukui, K.; Kuramochi, H. Effects of Microwave Irradiation on Triglyceride Transesterification: Experimental and Theoretical Studies. Biochem. Eng. J. 2011, 58–59, 20–24. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, L.; Li, H.; Ma, Y.; Zhang, R. High Selective Production of 5-Hydroxymethylfurfural from Fructose by Sulfonic Acid Functionalized SBA-15 Catalyst. Compos. Part B Eng. 2019, 156, 88–94. [Google Scholar] [CrossRef]
- Ji, T.; Tu, R.; Li, L.; Mu, L.; Liu, C.; Lu, X.; Zhu, J. Environmental Localizing Microwave Heat by Surface Polarization of Titanate Nanostructures for Enhanced Catalytic Reaction Efficiency. Appl. Catal. B Environ. 2018, 227, 266–275. [Google Scholar] [CrossRef]
- Qi, X.; Watanabe, M.; Aida, T.M.; Smith, R.L. Catalytic Dehydration of Fructose into 5-Hydroxymethylfurfural by Ion-Exchange Resin in Mixed-Aqueous System by Microwave Heating. Green Chem. 2008, 10, 799–805. [Google Scholar] [CrossRef]
- Lee, Y.; Lee, C.; Yoon, J. Kinetics and Mechanisms of DMSO (Dimethylsulfoxide) Degradation by UV/H2O2 Process. Water Res. 2004, 38, 2579–2588. [Google Scholar] [CrossRef]
- Tudino, T.C.; Nunes, R.S.; Mandelli, D.; Carvalho, W.A. Influence of Dimethylsulfoxide and Dioxygen in the Fructose Conversion to 5-Hydroxymethylfurfural Mediated by Glycerol’s Acidic Carbon. Front. Chem. 2020, 8, 263. [Google Scholar] [CrossRef] [PubMed]
- Whitaker, M.R.; Parulkar, A.; Ranadive, P.; Joshi, R.; Brunelli, N.A. Examining Acid Formation during the Selective Dehydration of Fructose to 5-hydroxymethylfurfural in Dimethyl Sulfoxide and Water. ChemSusChem 2019, 12, 2211–2219. [Google Scholar] [CrossRef]
- Hu, X.; Lievens, C.; Larcher, A.; Li, C.Z. Reaction Pathways of Glucose during Esterification: Effects of Reaction Parameters on the Formation of Humin Type Polymers. Bioresour. Technol. 2011, 102, 10104–10113. [Google Scholar] [CrossRef]
- Van de Steene, E.; De Clercq, J.; Thybaut, J.W. Ion-Exchange Resin Catalyzed Transesterification of Ethyl Acetate with Methanol: Gel versus Macroporous Resins. Chem. Eng. J. 2014, 242, 170–179. [Google Scholar] [CrossRef]
- Antonetti, C.; Fulignati, S.; Licursi, D.; Maria, A.; Galletti, R. Turning Point towards the Sustainable Production of HMF in Water: Metal Salts for Its Synthesis from Fructose and Inulin. ACS Sustain. Chem. Eng. 2019, 7, 6830–6838. [Google Scholar] [CrossRef]
- Esmaeili, N.; Zohuriaan-Mehr, M.J.; Bouhendi, H.; Bagheri-Marandi, G. HMF Synthesis in Aqueous and Organic Media under Ultrasonication, Microwave Irradiation and Conventional Heating. Korean J. Chem. Eng. 2016, 33, 1964–1970. [Google Scholar] [CrossRef]
- Kunov-kruse, A.J.; Riisager, A.; Saravanamurugan, S.; Berg, R.W.; Fehrmann, R. Revisiting the Brønsted Acid Catalysed Hydrolysis Kinetics of Polymeric Carbohydrates in Ionic Liquids by in Situ ATR-FTIR Spectroscopy. Green Chem. 2013, 15, 2843–2848. [Google Scholar] [CrossRef] [Green Version]
- Yang, F.; Tong, X.; Xia, F.; Zheng, C.; Qin, L.; Jiang, X. Efficient Hydroxymethylfurfural Production over Phosphoric Carbon Solid Acids. Catal. Lett. 2018, 148, 1848–1855. [Google Scholar] [CrossRef]
- Ferrari, A.; Hunt, J.; Lita, A.; Ashley, B.; Stiegman, A.E. Microwave-Specific Effects on the Equilibrium Constants and Thermodynamics of the Steam–carbon and Related Reactions. J. Phys. Chem. C 2014, 118, 9346–9356. [Google Scholar] [CrossRef]
- Priecel, P.; Lopez-Sanchez, J.A. Advantages and Limitations of Microwave Reactors: From Chemical Synthesis to the Catalytic Valorization of Biobased Chemicals. ACS Sustain. Chem. Eng. 2019, 7, 3–21. [Google Scholar] [CrossRef] [Green Version]
- Nayak, S.N.; Nayak, M.G.; Bhasin, D.C.P. Parametric, Kinetic, and Thermodynamic Studies of Microwave-Assisted Esterification of Kusum Oil. Fuel Commun. 2021, 8, 100018. [Google Scholar] [CrossRef]
- Perreux, L.; Loupy, A. A Tentative Rationalization of Microwave Effects in Organic Synthesis According to the Reaction Medium, and Mechanistic Considerations. Tetrahedron 2001, 57, 9199–9223. [Google Scholar] [CrossRef]
- Saha, B.; Abu-Omar, M.M. Advances in 5-Hydroxymethylfurfural Production from Biomass in Biphasic Solvents. Green Chem. 2013, 16, 24–38. [Google Scholar] [CrossRef]
- Qi, X.; Watanabe, M.; Aida, T.M.; Smith, R.L. Selective Conversion of D-Fructose to 5-Hydroxymethylfurfural by Ion-Exchange Resin in Acetone/Dimethyl Sulfoxide Solvent Mixtures. Ind. Eng. Chem. Res. 2008, 30, 9234–9239. [Google Scholar] [CrossRef]
- Moreau, C.; Durand, R.; Razigade, S.; Duhamet, J.; Faugeras, P.; Rivalier, P.; Pierre, R.; Avignon, G. Dehydration of Fructose to 5-Hydroxymethylfurfural over H-Mordenites. Appl. Catal. A Gen. 1996, 145, 211–224. [Google Scholar] [CrossRef]
- Villanueva, N.I.; Marzialetti, T.G. Mechanism and Kinetic Parameters of Glucose and Fructose Dehydration to 5-Hydroxymethylfurfural over Solid Phosphate Catalysts in Water. Catal. Today 2018, 302, 100–107. [Google Scholar] [CrossRef]
- Floris, B.; Sabuzi, F.; Galloni, P.; Conte, V. The Beneficial Sinergy of MW Irradiation and Ionic Liquids in Catalysis of Organic Reactions. Catalysts 2017, 7, 261. [Google Scholar] [CrossRef] [Green Version]
- Qi, X.; Watanabe, M.; Aida, T.M.; Smith, R.L. Efficient Process for Conversion of Fructose to 5-Hydroxymethylfurfural with Ionic Liquids. Green Chem. 2009, 11, 1327–1331. [Google Scholar] [CrossRef]
- Nunes, R.S.; Reis, G.M.; Vieira, L.M.; Mandelli, D.; Carvalho, W.A. Ultra-Fast Selective Fructose Dehydration Promoted by a Kraft Lignin Sulfonated Carbon Under Microwave Heating. Catal. Lett. 2021, 151, 398–408. [Google Scholar] [CrossRef]
- Qin, J.S.; Yuan, S.; Lollar, C.; Pang, J.; Alsalme, A.; Zhou, H.C. Stable Metal-Organic Frameworks as a Host Platform for Catalysis and Biomimetics. Chem. Commun. 2018, 54, 4231–4249. [Google Scholar] [CrossRef]
- CEM. CEM Operation Manual. In CEM Corporation Operation Manual; CEM: Charlotte, NC, USA, 2006. [Google Scholar]
- Kittrell, J.R. Mathematical Modeling of Chemical Reactions. In Advances in Chemical Engineering; Elsevier: Amsterdam, The Netherlands, 1970; Volume 8, pp. 97–183. [Google Scholar]
- Stewart, W.E.; Caracotsios, M. Computer-Aided Modelling of Reactive Systems. In Computer-Aided Modelling of Reactive Systems; John Wiley & Sons: Hoboken, NJ, USA, 2008; p. 141. ISBN 9780470274958. [Google Scholar]











| Catalyst Type | S Content [mmol g−1] | Fructose Conv. [%] | Yield [%] | Selectivity [%] | ||||
|---|---|---|---|---|---|---|---|---|
| 5-HMF | FA | LA | 5-HMF | FA | LA | |||
| MIL-101(Cr) | - | 99 | 9 | 0 | 0 | 9 | 0 | 0 |
| MIL-101(Cr)-SO3H(1) | 0.23 | 98 | 38 | 0 | 1 | 39 | 0 | 0 |
| MIL-101(Cr)-SO3H(2) | 0.56 | 99 | 54 | 2 | 1 | 55 | 2 | 0 |
| MIL-101(Cr)-SO3H(3) | 0.91 | 98 | 61 | 14 | 13 | 62 | 14 | 13 |
| Solvent (Tb °C) | Dielectric Constant (ε) | Dielectric Loss (ε″) | Tangent Delta (δ) |
|---|---|---|---|
| DMSO (189) | 45.0 | 37.1 | 0.825 |
| Acetone (56) | 20.7 | 1.1 | 0.054 |
| Temperature (°C) | Type of Heating | Time [min] | Fructose Conv. [%] | Yield [%] | ||
|---|---|---|---|---|---|---|
| 5-HMF | FA | LA | ||||
| 150 | MW | 5 | >99 | 0 | 0 | 0 |
| 160 | MW | 5 | >99 | 0 | 0 | 0 |
| 170 | MW | 5 | >99 | trace | trace | trace |
| 160 | CH | 60 | >99 | 0 | 0 | 0 |
| Catalyst Wt. [mg] | Time [min] | Solvent | Fructose Conv. [%] | Yield [%] | ||
|---|---|---|---|---|---|---|
| 5-HMF | FA | LA | ||||
| 30 | 50 | DMSO | 98 | 36 | 22 | 39 |
| 30 | 50 | DMSO/Acetone 70:30 | 98 | 48 | 17 | 25 |
| 10 | 5 | DMSO/Acetone 70:30 | >99 | 61 | 14 | 13 |
| 10 | 5 | DMSO/Acetone 60:40 | >99 | 11 | trace | trace |
| 10 | 5 | DMSO/Acetone 50:50 | >99 | 8 | trace | trace |
| 10 | 5 | DMSO/Acetone 30:70 | >99 | 0 | 0 | 0 |
| Method of Heating | Temperature [°C] | Batch Time [g·s] | Catalyst [mg] | Fructose Conv. [%] | 5-HMF Yield [%] |
|---|---|---|---|---|---|
| MW | 160 | 3 | 10 | 95 | 61 |
| CH | 160 | 36 | 10 | >99 | 60 |
| Reaction Step | Entropy of Activation, ΔSi [J/molK] | Activation Energy, Ei,a [kJ/mol] |
|---|---|---|
| Fru → Int. | −17.7 ± 1.4 | 88.0 ± 33.0 |
| Int. → 5-HMF | −25.8 ± 1.6 | 103.7 ± 43.0 |
| 5-HMF → LA + FA | −63.2 ± 1.9 | 87.8 ± 45.0 |
| 5-HMF → Humins | −38.2 ± 1.0 | - |
| Microwave effect (ΔSMW, ΔHMW) | 16.8 ± 1.3 | −87.5 ± 46.9 |
| Reaction Step | Conventional Heating [mmol/min] | Microwave Conditions [mmol/min] |
|---|---|---|
| Fru → Int. | (1.0 ± 0.002) × 10−1 | (8.0 ± 0.3) × 10−1 |
| Int. → 5-HMF | (4.6 ± 0.3) × 10−2 | (3.3 ± 0.22) × 10−1 |
| 5-HMF → LA + FA | (5.0 ± 0.8) × 10−3 | (3.3 ± 7.4) × 10−3 |
| 5-HMF → Humins | (9.0 ± 0.3) × 10−3 | (4.6 ± 0.43) × 10−2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aljammal, N.; Lauwaert, J.; Biesemans, B.; Verpoort, F.; Heynderickx, P.M.; Thybaut, J.W. Quantification of the Microwave Effect in the Synthesis of 5-Hydroxymethylfurfural over Sulfonated MIL-101(Cr). Catalysts 2023, 13, 622. https://doi.org/10.3390/catal13030622
Aljammal N, Lauwaert J, Biesemans B, Verpoort F, Heynderickx PM, Thybaut JW. Quantification of the Microwave Effect in the Synthesis of 5-Hydroxymethylfurfural over Sulfonated MIL-101(Cr). Catalysts. 2023; 13(3):622. https://doi.org/10.3390/catal13030622
Chicago/Turabian StyleAljammal, Noor, Jeroen Lauwaert, Bert Biesemans, Francis Verpoort, Philippe M. Heynderickx, and Joris W. Thybaut. 2023. "Quantification of the Microwave Effect in the Synthesis of 5-Hydroxymethylfurfural over Sulfonated MIL-101(Cr)" Catalysts 13, no. 3: 622. https://doi.org/10.3390/catal13030622