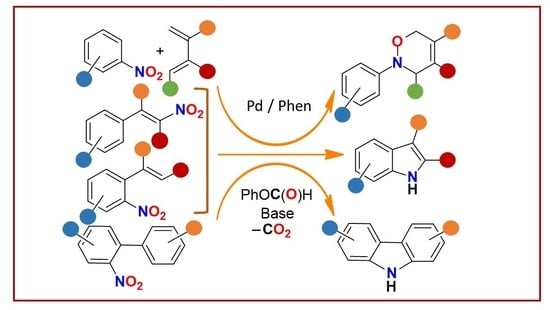
Phenyl Formate as a CO Surrogate for the Reductive Cyclization of Organic Nitro Compounds to Yield Different N-Heterocycles: No Need for Autoclaves and Pressurized Carbon Monoxide †
Abstract
:1. Introduction
2. Discussion
2.1. General Aspects
- The initial activation of the nitro compound, at least when late transition metal catalysts are employed, is always an electron transfer from the metal to the nitro group [22,23,24,25,26,27,28,29,30,31]. For this reason, low-valent metal complexes need to be used. However, due to the high sensitivity of the latter, metal complexes in higher oxidation states are often used as precatalysts, which are reduced by CO under the operating conditions. By the same token, nitroarene and to a lesser extent nitroalkenes are suitable substrates, but nitroalkanes have higher oxidation potentials and are unreactive in these systems.
- Palladium, ruthenium and rhodium compounds have all been employed as catalysts, but the best results have been obtained by the use of palladium and in the last decade the other two metals have only rarely been used.
- Phosphines have been used as ligands for palladium in many cases, but it has been shown that they are oxidized to phosphine oxides during the reaction [32]. Since we aim at developing a catalytic system that may also be applied at an industrial level, we prefer to avoid using them. No successful use of N-heterocyclic carbenes as ligands in this field has ever been reported. The best ligands in terms of activity and stability of the catalytic system are phenanthroline and its substituted derivatives [33,34,35,36].
- Aryl formates can be decomposed to CO and phenols even by weak organic bases. Alkyl formates are cheaper, but they are activated only by very strong bases, which would not be compatible with most reactions. Alternatively, they can be decomposed by the action of a ruthenium-based catalytic system [37].
- When using CO surrogates, the features of the vessel in which the reaction is performed are important for the success of the reaction and for safety reasons. We have discussed the pros and cons of different kinds of “pressure tubes” in a previous paper, thus we will not do it here again [38].
2.2. Synthesis of Indoles from O-Nitrostyrenes
2.3. Synthesis of Indoles from β-Nitrostyrenes
2.4. Synthesis of 3,6-dihydro-2H-[1,2]-oxazines from Nitroarenes and Conjugated Dienes
2.5. Synthesis of Carbazoles from o-Nitrobiphenyls
2.6. Future Perspectives
3. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vitaku, E.; Smith, D.T.; Njardarson, J.T. Analysis of the Structural Diversity, Substitution Patterns, and Frequency of Nitrogen Heterocycles among U.S. FDA Approved Pharmaceuticals. J. Med. Chem. 2014, 57, 10257–10274. [Google Scholar] [CrossRef] [PubMed]
- Ameta, K.L.; Kant, R.; Penoni, A.; Maspero, A.; Scapinello, L. (Eds.) N-Heterocycles; Springer: Singapore, 2022. [Google Scholar]
- Soderberg, B.C.G. Synthesis of heterocycles via intramolecular annulation of nitrene intermediates. Curr. Org. Chem. 2000, 4, 727–764. [Google Scholar] [CrossRef]
- Cenini, S.; Ragaini, F. Catalytic Reductive Carbonylation of Organic Nitro Compounds; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1996. [Google Scholar]
- Ragaini, F.; Cenini, S.; Gallo, E.; Caselli, A.; Fantauzzi, S. Fine chemicals by reductive carbonylation of nitroarenes, catalyzed by transition metal complexes. Curr. Org. Chem. 2006, 10, 1479–1510. [Google Scholar] [CrossRef]
- Ferretti, F.; Formenti, D.; Ragaini, F. The reduction of organic nitro compounds by carbon monoxide as an effective strategy for the synthesis of N-heterocyclic compounds: A personal account. Rend. Fis. Acc. Lincei 2017, 28, 97–115. [Google Scholar] [CrossRef]
- Afanasyev, O.I.; Chusov, D. Carbon Monoxide Related Reductions. Ineos Open 2020, 3, 133–139. [Google Scholar] [CrossRef]
- Tsygankov, A.A.; Makarova, M.; Chusov, D. Carbon monoxide as a selective reducing agent in organic chemistry. Mendeleev Commun. 2018, 28, 113–122. [Google Scholar] [CrossRef]
- Ferretti, F.; Ramadan, D.R.; Ragaini, F. Transition Metal Catalyzed Reductive Cyclization Reactions of Nitroarenes and Nitroalkenes. ChemCatChem 2019, 11, 4450–4488. [Google Scholar] [CrossRef]
- Peng, J.B.; Qi, X.X.; Wu, X.F. Recent Achievements in Carbonylation Reactions: A Personal Account. Synlett 2017, 28, 175–194. [Google Scholar]
- Wu, L.; Liu, Q.; Jackstell, R.; Beller, M. Carbonylations of Alkenes with CO Surrogates. Angew. Chem. Int. Ed. 2014, 53, 6310–6320. [Google Scholar] [CrossRef]
- Konishi, H.; Manabe, K. Formic Acid Derivatives as Practical Carbon Monoxide Surrogates for Metal-Catalyzed Carbonylation Reactions. Synlett 2014, 25, 1971–1986. [Google Scholar] [CrossRef]
- Gautam, P.; Bhanage, B.M. Recent advances in the transition metal catalyzed carbonylation of alkynes, arenes and aryl halides using CO surrogates. Catal. Sci. Technol. 2015, 5, 4663–4702. [Google Scholar] [CrossRef]
- Friis, S.D.; Lindhardt, A.T.; Skrydstrup, T. The Development and Application of Two-Chamber Reactors and Carbon Monoxide Precursors for Safe Carbonylation Reactions. Acc. Chem. Res. 2016, 49, 594–605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, F.; Wang, D.-S.; Driver, T.G. Palladium-Catalyzed Formation of N-Heteroarenes from Nitroarenes using Molybdenum Hexacarbonyl as the Source of Carbon Monoxide. Adv. Synth. Catal. 2015, 357, 3463–3468. [Google Scholar] [CrossRef]
- Su, Z.; Liu, B.; Liao, H.; Lin, H.-W. Synthesis of N-Heterocycles by Reductive Cyclization of Nitroalkenes Using Molybdenum Hexacarbonyl as Carbon Monoxide Surrogate. Eur. J. Org. Chem. 2020, 2020, 4059–4066. [Google Scholar] [CrossRef]
- Jana, N.; Zhou, F.; Driver, T.G. Promoting Reductive Tandem Reactions of Nitrostyrenes with Mo(CO)6 and a Palladium Catalyst To Produce 3H-Indoles. J. Am. Chem. Soc. 2015, 137, 6738–6741. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Wei, P.; Ying, J.; Wu, X.-F. Nickel-catalyzed carbonylative domino cyclization of arylboronic acid pinacol esters with 2-alkynyl nitroarenes toward N-aroyl indoles. Org. Chem. Front. 2022, 9, 2685–2689. [Google Scholar] [CrossRef]
- Yao, L.; Ying, J.; Wu, X.-F. Nickel-catalyzed cascade carbonylative synthesis of N-benzoyl indoles from 2-nitroalkynes and aryl iodides. Org. Chem. Front. 2021, 8, 6541–6545. [Google Scholar] [CrossRef]
- Qi, X.; Zhou, R.; Peng, J.-B.; Ying, J.; Wu, X.-F. Selenium-Catalyzed Carbonylative Synthesis of 2-Benzimidazolones from 2-Nitroanilines with TFBen as the CO Source. Eur. J. Org. Chem. 2019, 2019, 5161–5164. [Google Scholar] [CrossRef]
- Zhou, R.; Qi, X.; Wu, X.-F. Selenium-Catalyzed Carbonylative Synthesis of 3,4-Dihydroquinazolin-2(1H)-one Derivatives with TFBen as the CO Source. ACS Comb. Sci. 2019, 21, 573–577. [Google Scholar] [CrossRef]
- Berman, R.S.; Kochi, J.K. Kinetics and mechanism of oxygen atom transfer from nitro compounds mediated by nickel(0) complexes. Inorg. Chem. 1980, 19, 248–254. [Google Scholar] [CrossRef]
- Skoog, S.J.; Gladfelter, W.L. Activation of Nitroarenes in the Homogenous Catalytic Carbonylation of Nitroaromatics via an Oxygen-Atom-Transfer Mechanism Induced by Inner Sphere Electron Transfer. J. Am. Chem. Soc. 1997, 119, 11049–11060. [Google Scholar] [CrossRef]
- Kunin, A.J.; Noirot, M.D.; Gladfelter, W.L. In situ FTIR spectroscopy at elevated carbon monoxide pressure. Evidence for single electron transfer in the catalytic carbonylation of nitroaromatics. J. Am. Chem. Soc. 1989, 111, 2739–2741. [Google Scholar] [CrossRef]
- Belousov, Y.A. Radical chemistry of iron carbonyls. Russ. Chem. Rev. 2007, 76, 41–58. [Google Scholar] [CrossRef]
- Belousov, Y.A.; Kolosova, T.A. Electron-spin resonance study of the reaction of iron carbonyls with nitro and nitrosoparaffines—A mechanism of the reductive carbonylation of nitrocompounds. Polyhedron 1987, 6, 1959–1970. [Google Scholar] [CrossRef]
- Ragaini, F.; Song, J.S.; Ramage, D.L.; Geoffroy, G.L.; Yap, G.A.P.; Rheingold, A.L. Radical Processes in the Reduction of Nitrobenzene Promoted by Iron Carbonyl Clusters—X-ray Crystal-Structures of [Fe3(CO)9(μ3-NPh)]2−, [HFe3(CO)9(μ3-NPh)]−, and the Radical-Anion [Fe3(CO)11]−. Organometallics 1995, 14, 387–400. [Google Scholar] [CrossRef]
- Ragaini, F. Mechanistic study of the phase-transfer-catalyzed reduction of nitrobenzene to aniline by iron carbonyl complexes. Role of the radical anion [Fe3(CO)11)]−. Organometallics 1996, 15, 3572–3578. [Google Scholar] [CrossRef]
- Liu, P.H.; Liao, H.Y.; Cheng, C.H. Electro-transfer from tetracarbonylrhodate(1-) to nitro aromatics—Novel interaction of nitro radical-anions with a rhodium(I) center. J. Chem. Soc. Chem. Commun. 1995, 23, 2441–2442. [Google Scholar] [CrossRef] [Green Version]
- Ragaini, F.; Cenini, S.; Demartin, F. Mechanistic Studies of the Carbonylation of Nitrobenzene Catalyzed by the [Rh(CO)4]−/Bipy System—X-ray Structure of [PPN][Rh(CO)2ON[C6H3Cl2)C(O)O] [PPN+=(PPh3)2N+-Bipy = 2,2’-Bipyridyl]. J. Chem. Soc. Chem. Commun. 1992, 1467–1468. [Google Scholar] [CrossRef]
- Ragaini, F.; Cenini, S.; Demartin, F. Mechanistic Study of the Carbonylation of Nitrobenzene Catalyzed by the [Rh(CO)4]− Nitrogen Base System—X-ray Structure of [PPN][Rh(CO)2ON(C6H3Cl2)C(O)O]. Organometallics 1994, 13, 1178–1189. [Google Scholar] [CrossRef]
- Wehman, P.; van Donge, H.M.A.; Hagos, A.; Kamer, P.C.J.; van Leeuwen, P.W.N.M. Influence of various P/N and P/P ligands on the palladium-catalysed reductive carbonylation of nitrobenzene. J. Organomet. Chem. 1997, 535, 183–193. [Google Scholar] [CrossRef]
- Bontempi, A.; Alessio, E.; Chanos, G.; Mestroni, G. Reductive carbonylation of nitroaromatic compounds to urethanes catalyzed by (di-1,10-phenanthroline)palladium bis(hexafluorophosphate) and related complexes. J. Mol. Catal. 1987, 42, 67–80. [Google Scholar] [CrossRef]
- Wehman, P.; Kaasjager, V.E.; Delange, W.G.J.; Hartl, F.; Kamer, P.C.J.; van Leeuwen, P.W.N.M.; Fraanje, J.; Goubitz, K. Subtle Balance between Various Phenanthroline Ligands and Anions in the Palladium-Catalyzed Reductive Carbonylation of Nitrobenzene. Organometallics 1995, 14, 3751–3761. [Google Scholar] [CrossRef] [Green Version]
- Ferretti, F.; Gallo, E.; Ragaini, F. Nitrogen ligands effects in the palladium-catalyzed carbonylation reaction of nitrobenzene to give N-methyl phenylcarbamate. J. Organomet. Chem. 2014, 771, 59–67. [Google Scholar] [CrossRef]
- Ferretti, F.; Ragaini, F.; Lariccia, R.; Gallo, E.; Cenini, S. New Nonsymmetric Phenanthrolines as Very Effective Ligands in the Palladium-Catalyzed Carbonylation of Nitrobenzene. Organometallics 2010, 29, 1465–1471. [Google Scholar] [CrossRef]
- Jenner, G.; Nahmed, E.M.; Leismann, H. Homogeneous Activation of th C-H Bond in Formates—Decarbonylation of Formates to Alcohols. J. Organomet. Chem. 1990, 387, 315–321. [Google Scholar] [CrossRef]
- Fouad, M.A.; Ferretti, F.; Formenti, D.; Milani, F.; Ragaini, F. Synthesis of Indoles by Reductive Cyclization of Nitro Compounds Using Formate Esters as CO Surrogates. Eur. J. Org. Chem. 2021, 2021, 4876–4894. [Google Scholar] [CrossRef]
- Cenini, S.; Crotti, C.; Pizzotti, M.; Porta, F. Ruthenium carbonyl catalyzed reductive carbonylation of aromatic nitro compounds. A selective route to carbamates. J. Org. Chem. 1988, 53, 1243–1250. [Google Scholar] [CrossRef]
- Cenini, S.; Pizzotti, M.; Crotti, C.; Ragaini, F.; Porta, F. Effects of neutral ligands in the reductive carbonylation of nitrobenzene catalyzed by Ru3(CO)12 and Rh6(CO)16. J. Mol. Catal. 1988, 49, 59–69. [Google Scholar] [CrossRef]
- Ben Taleb, A.; Jenner, G. Catalytic reduction of nitrobenzene to aniline with aqueous methyl formate. J. Organomet. Chem. 1993, 456, 263–269. [Google Scholar] [CrossRef]
- Formenti, D.; Ferretti, F.; Ragaini, F. Synthesis of N-Heterocycles by Reductive Cyclization of Nitro Compounds using Formate Esters as Carbon Monoxide Surrogates. ChemCatChem 2018, 10, 148–152. [Google Scholar] [CrossRef]
- Gasperini, M.; Ragaini, F.; Cazzaniga, C.; Cenini, S. Carbonylation of dinitrotoluene to dimethyl toluenedicarbamate; high efficiency of phosphorus acids as promoters for the palladium-phenanthroline catalytic system. Adv. Synth. Catal. 2005, 347, 105–120. [Google Scholar] [CrossRef]
- Oh, J.S.; Lee, S.M.; Yeo, J.K.; Lee, C.W.; Lee, J.S. Palladium-Catalyzed Synthesis of N,N′-Diphenylurea from Nitrobenzene, Aniline, and Carbon Monoxide. Ind. Eng. Chem. Res. 1991, 30, 1456–1461. [Google Scholar] [CrossRef]
- Gasperini, M.; Ragaini, F.; Remondini, C.; Caselli, A.; Cenini, S. The palladium-phenanthroline catalyzed carbonylation of nitroarenes to diarylureas: Effect of chloride and diphenylphosphinic acid. J. Organomet. Chem. 2005, 690, 4517–4529. [Google Scholar] [CrossRef]
- Hsieh, T.H.H.; Dong, V.M. Indole synthesis: Palladium-catalyzed C-H bond amination via reduction of nitroalkenes with carbon monoxide. Tetrahedron 2009, 65, 3062–3068. [Google Scholar] [CrossRef]
- Ferretti, F.; El-Atawy, M.A.; Muto, S.; Hagar, M.; Gallo, E.; Ragaini, F. Synthesis of Indoles by Palladium-Catalyzed Reductive Cyclization of β-Nitrostyrenes with Carbon Monoxide as the Reductant. Eur. J. Org. Chem. 2015, 2015, 5712–5715. [Google Scholar] [CrossRef]
- El-Atawy, M.A.; Ferretti, F.; Ragaini, F. Palladium-Catalyzed Intramolecular Cyclization of Nitroalkenes: Synthesis of Thienopyrroles. Eur. J. Org. Chem. 2017, 2017, 1902–1910. [Google Scholar] [CrossRef]
- EL-Atawy, M.A.; Ferretti, F.; Ragaini, F. A Synthetic Methodology for Pyrroles from Nitrodienes. Eur. J. Org. Chem. 2018, 2018, 4818–4825. [Google Scholar] [CrossRef]
- Ferretti, F.; Fouad, M.A.; Ragaini, F. Synthesis of Indoles by Palladium-Catalyzed Reductive Cyclization of Nitrostyrenes with Phenyl Formate as a CO Surrogate. Catalysts 2022, 12, 106. [Google Scholar] [CrossRef]
- Boger, D.L.; Weinreb, S.M. Hetero Diels-Alder Methodology in Organic Synthesis; Academic Press: New York, NY, USA, 1987. [Google Scholar]
- Carosso, S.; Miller, M.J. Nitroso Diels-Alder (NDA) Reaction as an Efficient Tool for the Functionalization of Diene-Containing Natural Products. Org. Biomol. Chem. 2014, 12, 7445–7468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maji, B.; Yamamoto, H. Catalytic Enantioselective Nitroso Diels-Alder Reaction. J. Am. Chem. Soc. 2015, 137, 15957–15963. [Google Scholar] [CrossRef]
- Ragaini, F.; Cenini, S.; Brignoli, D.; Gasperini, M.; Gallo, E. Synthesis of oxazines and N-arylpyrroles by reaction of unfunctionalized dienes with nitroarenes and carbon monoxide, catalyzed by palladium-phenanthroline complexes. J. Org. Chem. 2003, 68, 460–466. [Google Scholar] [CrossRef] [PubMed]
- Ragaini, F.; Cenini, S.; Borsani, E.; Dompe, M.; Gallo, E.; Moret, M. Synthesis of N-arylpyrroles, hetero-Diels-Alder adducts, and allylic amines by reaction of unfunctionalized dienes with nitroarenes and carbon monoxide, catalyzed by Ru(CO)3(Ar-BIAN). Organometallics 2001, 20, 3390–3398. [Google Scholar] [CrossRef]
- EL-Atawy, M.A.; Formenti, D.; Ferretti, F.; Ragaini, F. Synthesis of 3,6-Dihydro-2H-[1,2]-Oxazines from Nitroarenes and Conjugated Dienes, Catalyzed by Palladium/Phenanthroline Complexes and Employing Phenyl Formate as a CO Surrogate. ChemCatChem 2018, 10, 4707–4717. [Google Scholar] [CrossRef] [Green Version]
- Crotti, C.; Cenini, S.; Bassoli, A.; Rindone, B.; Demartin, F. Synthesis of carbazole by triruthenium dodecacarbonyl catalyzed reductive carbonylation of 2-nitrobiphenyl: The crystal and molecular structure of Ru3(μ3-NC6H4-o-C6H5)2(CO)9. J. Mol. Catal. 1991, 70, 175–187. [Google Scholar] [CrossRef]
- Smitrovich, J.H.; Davies, I.W. Catalytic C-H functionalization driven by CO as a stoichiometric reductant: Application to carbazole synthesis. Org. Lett. 2004, 6, 533–535. [Google Scholar] [CrossRef]
- Ramadan, D.R.; Ferretti, F.; Ragaini, F. Catalytic reductive cyclization of 2-nitrobiphenyls using phenyl formate as CO surrogate: A robust synthesis of 9H-carbazoles. J. Catal. 2022, 409, 41–47. [Google Scholar] [CrossRef]
- Cenini, S.; Bettettini, E.; Fedele, M.; Tollari, S. Intramolecular amination catalyzed by ruthenium and palladium. Synthesis of 2-acyl indoles and 2-aryl quinolines by carbonylation of 2-nitrochalcones. J. Mol. Catal. A Chem. 1996, 111, 37–41. [Google Scholar] [CrossRef]
- Ragaini, F.; Sportiello, P.; Cenini, S. Investigation of the possible role of arylamine formation in the ortho-substituted nitroarenes reductive cyclization reactions to afford heterocycles. J. Organomet. Chem. 1999, 577, 283–291. [Google Scholar] [CrossRef]
- Fouad, M.A.; Ferretti, F.; Ragaini, F. Formic Acid as Carbon Monoxide Source in the Palladium-Catalyzed N-Heterocyclization of o-Nitrostyrenes to Indoles. J. Org. Chem. 2023, in press. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ragaini, F.; Ferretti, F.; Fouad, M.A. Phenyl Formate as a CO Surrogate for the Reductive Cyclization of Organic Nitro Compounds to Yield Different N-Heterocycles: No Need for Autoclaves and Pressurized Carbon Monoxide. Catalysts 2023, 13, 224. https://doi.org/10.3390/catal13020224
Ragaini F, Ferretti F, Fouad MA. Phenyl Formate as a CO Surrogate for the Reductive Cyclization of Organic Nitro Compounds to Yield Different N-Heterocycles: No Need for Autoclaves and Pressurized Carbon Monoxide. Catalysts. 2023; 13(2):224. https://doi.org/10.3390/catal13020224
Chicago/Turabian StyleRagaini, Fabio, Francesco Ferretti, and Manar Ahmed Fouad. 2023. "Phenyl Formate as a CO Surrogate for the Reductive Cyclization of Organic Nitro Compounds to Yield Different N-Heterocycles: No Need for Autoclaves and Pressurized Carbon Monoxide" Catalysts 13, no. 2: 224. https://doi.org/10.3390/catal13020224