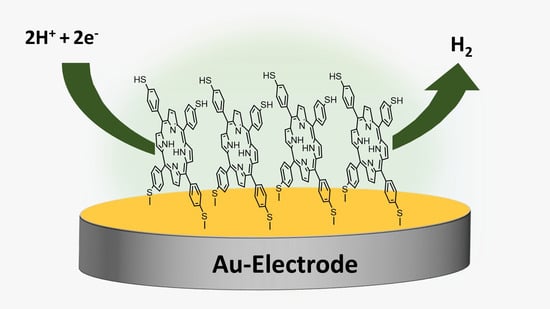
Hydrogen Gas Generation Using Self-Assembled Monolayers (SAMs) of 5,10,15,20-Tetrakis (p-Thiophenol) Porphyrin on a Gold Electrode
Abstract
:1. Introduction
2. Results and Discussion
2.1. Organic Chemistry
Synthesis and Characterization of 5,10,15,20-Tetrakis (p-Thiophenol) Porphyrin (TPTH-P) (2)
2.2. Electrochemistry
2.2.1. Formation and Characterization of the SAM on the Au Surface
2.2.2. Electrochemical Activity of the Au-TPTH-P Electrode
2.2.3. Electrochemical Activation of the Au-TPTH-P Electrode
2.2.4. The Effect of Immersion Time on Catalytic Activity
- (i)
- The adsorption of a proton on the catalytically active site (Tafel slope of 120 mV·dec−1).
- (ii)
- The electrochemical desorption of molecular hydrogen (Tafel slope ranging from 40 to 120 mV·dec−1).
- (iii)
- The chemical desorption of molecular hydrogen (Tafel slope ranging from 30 to 40 mV·dec−1).
2.2.5. Chronoamperometry
2.2.6. Proposed HER Mechanism at the Au-TPTH-P Electrode
3. Materials and Methods
3.1. Synthesis of 5,10,15,20-Tetrakis (p-Thiophenol) Porphyrin (2)
3.2. SAM Formation on the Gold Electrode
3.3. The Contact Angle Measurement
3.4. Electrochemical Measurements
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gooding, J.J.; Mearns, F.; Yang, W.; Liu, J. Self-Assembled Monolayers into the 21st Century: Recent Advances and Applications. Electroanalysis 2003, 15, 81–96. [Google Scholar] [CrossRef]
- Wang, L.; Schubert, U.S.; Hoeppener, S. Surface Chemical Reactions on Self-Assembled Silane Based Monolayers. Chem. Soc. Rev. 2021, 50, 6507–6540. [Google Scholar] [CrossRef] [PubMed]
- Nicosia, C.; Huskens, J. Reactive Self-Assembled Monolayers: From Surface Functionalization to Gradient Formation. Mater. Horiz. 2014, 1, 32–45. [Google Scholar] [CrossRef]
- Benneckendorf, F.S.; Rohnacher, V.; Sauter, E.; Hillebrandt, S.; Münch, M.; Wang, C.; Casalini, S.; Ihrig, K.; Beck, S.; Jänsch, D.; et al. Tetrapodal Diazatriptycene Enforces Orthogonal Orientation in Self-Assembled Monolayers. ACS Appl. Mater. Interfaces 2020, 12, 6565–6572. [Google Scholar] [CrossRef]
- Noomuna, P. Preparation and Characterization of Self-Assembled Monolayers and Mesoscale Protein Patterning. Master’s Thesis, Eastern Kentucky University, Nairobi, Kenya, 2015. [Google Scholar]
- Sinnott, S.B.; Heo, S.J.; Brenner, D.W.; Harrison, J.A.; Irving, D.L. Nanotribology and Nanomechanics: An Introduction, 4th ed.; Springer International Publishing: Berlin/Heidelberg, Germany, 2017. [Google Scholar] [CrossRef]
- Love, J.C.; Estroff, L.A.; Kriebel, J.K.; Nuzzo, R.G.; Whitesides, G.M. Self-Assembled Monolayers of Thiolates on Metals as a Form of Nanotechnology. Chem. Rev. 2005, 105, 1103–1170. [Google Scholar] [CrossRef] [PubMed]
- Lukkari, J.; Kleemola, K.; Meretoja, M.; Ollonqvist, T.; Kankare, J. Electrochemical Post-Self-Assembly Transformation of 4-Aminothiophenol Monolayers on Gold Electrodes. Langmuir 1998, 14, 1705–1715. [Google Scholar] [CrossRef]
- Mezour, M.A.; Cornut, R.; Hussien, E.M.; Morin, M.; Mauzeroll, J. Detection of Hydrogen Peroxide Produced during the Oxygen Reduction Reaction at Self-Assembled Thiol-Porphyrin Monolayers on Gold Using SECM and Nanoelectrodes. Langmuir 2010, 26, 13000–13006. [Google Scholar] [CrossRef]
- Zhang, Z.; Hou, S.; Zhu, Z.; Liu, Z. Preparation and Characterization of a Porphyrin Self-Assembled Monolayer with a Controlled Orientation on Gold. Langmuir 2000, 16, 537–540. [Google Scholar] [CrossRef]
- Bhushan, B. Self-Assembled Monolayers for Controlling Hydrophobicity and/or Friction and Wear. Mod. Tribol. Handb. Vol. One Princ. Tribol. 2000, 1, 909–929. [Google Scholar]
- Colangelo, E.; Comenge, J.; Paramelle, D.; Volk, M.; Chen, Q.; Lévy, R. Characterizing Self-Assembled Monolayers on Gold Nanoparticles. Bioconjugate Chem. 2017, 28, 11–22. [Google Scholar] [CrossRef]
- Chattopadhyay, S.; Sarkar, A.; Chatterjee, S.; Dey, A. Functional Adlayers on Au Electrodes: Some Recent Applications in Hydrogen Evolution and Oxygen Reduction. J. Mater. Chem. A 2018, 6, 1323–1339. [Google Scholar] [CrossRef]
- Lu, X.; Li, M.; Yang, C.; Zhang, L.; Li, Y.; Jiang, L.L.; Li, H.; Jiang, L.L.; Liu, C.; Hu, W. Electron Transport through a Self-Assembled Monolayer of Thiol-End-Functionalized Tetraphenylporphines and Metal Tetraphenylporphines. Langmuir 2006, 22, 3035–3039. [Google Scholar] [CrossRef]
- Verónica Rivas, M.; De Leo, L.P.M.; Hamer, M.; Carballo, R.; Williams, F.J. Self-Assembled Monolayers of Disulfide Cu Porphyrins on Au Surfaces: Adsorption Induced Reduction and Demetalation. Langmuir 2011, 27, 10714–10721. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Zhang, L.; Li, M.; Wang, X.; Zhang, Y.; Liu, X.; Zuo, G. Electrochemical Characterization of Self-Assembled Thiol-Porphyrin Monolayers on Gold Electrodes by SECM. ChemPhysChem 2006, 7, 854–862. [Google Scholar] [CrossRef]
- Elghamry, I.; Alablan, A.S.; Abdelsalam, M.E. A Bifunctional Electrocatalyst Based on Ni-Porphyrin/Vapor-Grown Carbon Fibres for Oxygen Reduction and Evolution Reactions in Alkaline Media. Int. J. Electrochem. Sci. 2022, 17, 221013. [Google Scholar] [CrossRef]
- Shang, X.; Chi, J.-Q.; Lu, S.-S.; Dong, B.; Li, X.; Liu, Y.-R.; Yan, K.-L.; Gao, W.-K.; Chai, Y.-M.; Liu, C.-G. Novel CoxSy/WS2 Nanosheets Supported on Carbon Cloth as Efficient Electrocatalyst for Hydrogen Evolution Reaction. Int. J. Hydrog. Energy 2017, 42, 4165–4173. [Google Scholar] [CrossRef]
- Hirsch, T.; Zharnikov, M.; Shaporenko, A.; Stahl, J.; Weiss, D.; Wolfbeis, O.S.; Mirsky, V.M. Size-Controlled Electrochemical Synthesis of Metal Nanoparticles on Monomolecular Templates. Angew. Chem. Int. Ed. 2005, 44, 6775–6778. [Google Scholar] [CrossRef]
- Oae, S.; Togo, H. Reduction of Sulfonic Acids with Triphenylphosphine–Diaryl Disulfide System. Bull. Chem. Soc. Jpn. 1984, 57, 232–236. [Google Scholar] [CrossRef]
- Macdonald, E.H. Handbook of Gold Exploration and Evaluation; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Biradha, K.; Goswami, A.; Moi, R. Coordination Polymers as Heterogeneous Catalysts in Hydrogen Evolution and Oxygen Evolution Reactions. Chem. Commun. 2020, 56, 10824–10842. [Google Scholar] [CrossRef]
- Spring, S.; Shinde, P.S.; Fontenot, P.R.; Donahue, J.P.; Pan, S. Self-Assembled Monolayers of Molybdenum Sulfide Clusters on Au Electrode as Hydrogen Evolution Catalyst for Solar Water Splitting. Inorganics 2019, 7, 79. [Google Scholar] [CrossRef]
- Manton, J.C.; Hidalgo, D.; Frayne, L.; Brandon, M.P.; Vos, J.G.; Pryce, M.T. Electrocatalytic Hydrogen Evolution Using Metal-Free Porphyrins. Int. J. Hydrog. Energy 2018, 43, 18843–18849. [Google Scholar] [CrossRef]
- Huang, D.; Lu, J.; Li, S.; Luo, Y.; Zhao, C.; Hu, B.; Wang, M.; Shen, Y. Fabrication of Cobalt Porphyrin. Electrochemically Reduced Graphene Oxide Hybrid Films for Electrocatalytic Hydrogen Evolution in Aqueous Solution. Langmuir 2014, 30, 6990–6998. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Zhang, H.; Li, S.; Wang, R.; Sun, X.; Zhou, M.; Zhou, J.; Lou, X.W.; Xie, Y. Defect-Rich MoS2 Ultrathin Nanosheets with Additional Active Edge Sites for Enhanced Electrocatalytic Hydrogen Evolution. Adv. Mater. 2013, 25, 5807–5813. [Google Scholar] [CrossRef] [PubMed]
- Sukeri, A.; Bertotti, M. Nanoporous Gold Surface: An Efficient Platform for Hydrogen Evolution Reaction at Very Low Overpotential. J. Braz. Chem. Soc. 2018, 29, 226–231. [Google Scholar] [CrossRef]
- Wu, Y.; Rodríguez-López, N.; Villagrán, D. Hydrogen Gas Generation Using a Metal-Free Fluorinated Porphyrin. Chem. Sci. 2018, 9, 4689–4695. [Google Scholar] [CrossRef]















| Unmodified Au Electrode | Au-TPTH-P | |
|---|---|---|
| Rs/Ω.cm2 | 1.49 | 4.6 |
| Catalyst | ||||
|---|---|---|---|---|
| MeOTTP | Environment | η at 3 mA·cm−2 | Tafel Slope (b) | Ref |
| [ERGO@CoTMPyP] | pH 2.0/0.1 M NaH2PO4 | 562 mV | 111 mV·dec−1 | [24] |
| MoS2 | 0.5 M H2SO4 | 530 mV | NA | [25] |
| Pt/C | 0.5 M H2SO4 | 182 mV | 50 mV·dec−1 | [26] |
| TPTH-P | 0.5 M H2SO4 | 15.5 mV | 30 mV·dec−1 | [27] |
| * It is expected that increasing the H2SO4 concentration to 0.5 M would decrease both the b and η values. | 0.1* M H2SO4 | 127 mV | 81 mV·dec−1 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elghamry, I.; Alablan, A.S.; Abdelsalam, M.E. Hydrogen Gas Generation Using Self-Assembled Monolayers (SAMs) of 5,10,15,20-Tetrakis (p-Thiophenol) Porphyrin on a Gold Electrode. Catalysts 2023, 13, 1355. https://doi.org/10.3390/catal13101355
Elghamry I, Alablan AS, Abdelsalam ME. Hydrogen Gas Generation Using Self-Assembled Monolayers (SAMs) of 5,10,15,20-Tetrakis (p-Thiophenol) Porphyrin on a Gold Electrode. Catalysts. 2023; 13(10):1355. https://doi.org/10.3390/catal13101355
Chicago/Turabian StyleElghamry, Ibrahim, Abdulrahman S. Alablan, and Mamdouh E. Abdelsalam. 2023. "Hydrogen Gas Generation Using Self-Assembled Monolayers (SAMs) of 5,10,15,20-Tetrakis (p-Thiophenol) Porphyrin on a Gold Electrode" Catalysts 13, no. 10: 1355. https://doi.org/10.3390/catal13101355