Hydrogen Production via Methanol Steam Reforming over CuO/ZnO/Al2O3 Catalysts Prepared via Oxalate-Precursor Synthesis
Abstract
:1. Introduction
2. Results and Discussion
2.1. XRD Characterization of Precursors
2.2. DTG Characterization of Precursors
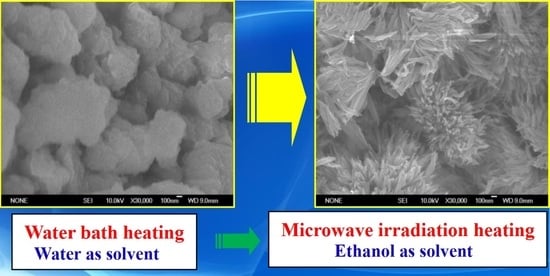
2.3. SEM Images of Precursors and Catalysts
2.4. XRD Characterization of Catalysts
2.5. H2-TPR Characterization of Catalysts
2.6. XPS and AES Characterization of Catalysts
2.7. Catalytic Performance Test for MSR Reaction
3. Experimental
3.1. Catalyst Preparation
3.2. Catalyst Characterization
3.3. Catalytic Performance of MSR
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Poudel, M.B.; Kim, H.J. Confinement of Zn-Mg-Al-layered double hydroxide and α-Fe2O3 nanorods on hollow porous carbon nanofibers: A free-standing electrode for solid-state symmetric supercapacitors. Chem. Eng. J. 2022, 429, 132345. [Google Scholar] [CrossRef]
- Kawamura, Y.; Ogura, N.; Igarashi, A. Hydrogen production by methanol steam reforming using microreactor. J. Jpn. Petrol. Inst. 2013, 56, 288–297. [Google Scholar] [CrossRef]
- Yong, S.T.; Ooi, C.W.; Chai, S.P.; Wu, X.S. Review of methanol reforming-Cu-based catalysts, surface reaction mechanisms, and reaction schemes. Int. J. Hydrogen Energy 2013, 38, 9541–9552. [Google Scholar] [CrossRef]
- Xu, X.; Shuai, K.; Xu, B. Review on copper and palladium based catalysts for methanol steam reforming to produce hydrogen. Catalysts 2017, 7, 183. [Google Scholar] [CrossRef]
- Iulianelli, A.; Ribeirinha, P.; Mendes, A.; Basile, A. Methanol steam reforming for hydrogen generation via conventional and membrane reactors, a review. Renew. Sustain. Energy Rev. 2014, 29, 355–368. [Google Scholar] [CrossRef]
- Poudel, M.B.; Lohani, P.C.; Acharya, D.; Kandel, D.; Kim, A.A.; Yoo, D.J. MOF derived hierarchical ZnNiCo-LDH on vapor solid phase grown CuxO nanowire array as high energy density asymmetric supercapacitors. J. Energy Storage 2023, 72, 108220. [Google Scholar] [CrossRef]
- Iwasa, N.; Masuda, S.; Ogawa, N.; Takezawa, N. Steam reforming of methanol over Pd/ZnO, effect of the formation of PdZn alloys upon the reaction. Appl. Catal. A 1995, 125, 145–157. [Google Scholar] [CrossRef]
- Shokrani, R.; Haghighi, M.; Jodeiri, N.; Ajamein, H.; Abdollahifar, M. Fuel cell grade hydrogen production via methanol steam reforming over CuO/ZnO/Al2O3 nanocatalyst with various oxide ratios synthesized via urea-nitrates combustion method. Int. J. Hydrogen Energy 2014, 39, 13141–13155. [Google Scholar] [CrossRef]
- Shishido, T.; Yamamoto, Y.; Morioka, H.; Takehira, K. Production of hydrogen from methanol over Cu/ZnO and Cu/ZnO/Al2O3 catalysts prepared by homogeneous precipitation: Steam reforming and oxidative steam reforming. J. Mol. Catal. A 2007, 268, 185–194. [Google Scholar] [CrossRef]
- Agrell, J.; Birgersson, H.; Boutonnet, M.; Melián-Cabrera, I.; Navarro, R.M.; Fierro, J.L.G. Production of hydrogen from methanol over Cu/ZnO catalysts promoted by ZrO2 and Al2O3. J. Catal. 2003, 219, 389–403. [Google Scholar] [CrossRef]
- Spencer, M.S. Precursors of copper/zinc oxide catalysts. Catal. Lett. 2000, 66, 255–257. [Google Scholar] [CrossRef]
- Li, J.; Inui, T. Characterization of precursors of methanol synthesis catalysts copper/zinc/aluminum oxides precipitated at different pH and temperatures. Appl. Catal. A 1996, 137, 105–117. [Google Scholar] [CrossRef]
- Fang, D.; Ren, W.; Liu, Z.; Xu, X.; Xu, L.; Lv, H.; Liao, W.; Zhang, H. Synthesis and applications of mesoporous Cu-Zn-Al2O3 catalyst for dehydrogenation of 2-butanol. J. Nat. Gas. Chem. 2009, 18, 179–182. [Google Scholar] [CrossRef]
- Ma, Y.; Sun, Q.; Wu, D.; Fan, W.; Zhang, Y.; Deng, J. A practical approach for the preparation of high activity Cu/ZnO/ZrO2 catalyst for methanol synthesis from CO2 hydrogenation. Appl. Catal. A 1998, 171, 45–55. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, Q.; Deng, J.; Wu, D.; Chen, S. A high activity Cu/ZnO/Al2O3 catalyst for methanol synthesis: Preparation and catalytic properties. Appl. Catal. A 1997, 158, 105–120. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, L.; Yao, C.; Cao, Y.; Dai, W.; He, H.; Fan, K. A highly efficient Cu/ZnO/Al2O3 catalyst via gel-coprecipitation of oxalate precursors for low-temperature steam reforming of methanol. Catal. Lett. 2005, 102, 183–190. [Google Scholar] [CrossRef]
- Dai, W.; Sun, Q.; Deng, J.; Wu, D.; Sun, Y. XPS studies of Cu/ZnO/Al2O3 ultra-fine catalysts derived by a novel gel oxalate co-precipitation for methanol synthesis by CO2+H2. Appl. Surf. Sci. 2001, 177, 172–179. [Google Scholar] [CrossRef]
- Wu, Z.; Ge, S.; Zhang, M.; Li, W.; Tao, K. Synthesis of a supported nickel boride catalyst under microwave irradiation. Catal. Commun. 2008, 9, 1432–1438. [Google Scholar] [CrossRef]
- Sule, E.E.; Sadik, C.; Siddik, I. Conventional and microwave-assisted synthesis of ZnO nanorods and effects of PEG400 as a surfactant on the morphology. Inorg. Chim. Acta 2009, 362, 1855–1858. [Google Scholar]
- Zhang, X.; Wang, L.; Cao, Y.; Dai, W.; He, H.; Fan, K. A unique microwave effect on the microstructural modification of Cu/ZnO/Al2O3 catalysts for steam reforming of methanol. Chem. Commun. 2005, 102, 4104–4106. [Google Scholar] [CrossRef]
- Fernández, Y.; Menéndez, J.; Arenillas, A.; Fuente, E.; Peng, J.; Zhang, Z.; Li, W.; Zhang, Z. Microwave-assisted synthesis of CuO/ZnO and CuO/ZnO/Al2O3 precursors using urea hydrolysis. Solid State Ion. 2009, 180, 1372–1378. [Google Scholar] [CrossRef]
- Nakamura, J.; Choi, Y.; Fujitani, T. On the issue of the active site and the role of ZnO in Cu/ZnO methanol synthesis catalysts. Top. Catal. 2003, 22, 277–285. [Google Scholar] [CrossRef]
- Kasatkin, I.; Kurr, P.; Kniep, B.; Trunschke, A.; Schlögl, R. Role of lattice strain and defects in copper particles on the activity of Cu/ZnO/Al2O3 catalysts for methanol synthesis. Angew. Chem. Int. Ed. 2007, 46, 7324–7327. [Google Scholar] [CrossRef] [PubMed]
- Behrens, M.; Studt, F.; Kasatkin, I.; Kühl, S.; Hävecker, M.; Abild-Pedersen, F. The active site of methanol synthesis over Cu/ZnO/Al2O3 industrial catalysts. Science 2012, 336, 893–897. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liu, Y.; Chen, M.; Cao, Y.; He, H.; Wu, G.; Dai, W.; Fan, K. Production of hydrogen by steam reforming of methanol over Cu/ZnO catalysts prepared via a practical soft reactive grinding route based on dry oxalate-precursor synthesis. J. Catal. 2007, 246, 193–204. [Google Scholar] [CrossRef]
- Ning, W.; Shen, H.; Liu, H. Study of the effect of preparation method on CuO-ZnO-Al2O3 Catalyst. Appl. Catal. A 2001, 211, 153–157. [Google Scholar] [CrossRef]
- An, X.; Li, J.; Zuo, Y.; Zhang, Q.; Wang, D.; Wang, J. A Cu/Zn/Al/Zr fibrous catalyst that is an improved CO2 hydrogenation to methanol catalyst. Catal. Lett. 2007, 118, 264–269. [Google Scholar] [CrossRef]
- Bao, J.; Liu, Z.; Zhang, Y.; Tsubaki, N. Preparation of mesoporous Cu/ZnO catalyst and its application in low-temperature methanol synthesis. Catal. Commun. 2008, 9, 913–918. [Google Scholar] [CrossRef]
- Yang, R.; Yu, X.; Zhang, Y.; Li, W.; Tsubaki, N. A new method of low-temperature methanol synthesis on Cu/ZnO/Al2O3 catalysts from CO/CO2/H2. Fuel 2008, 87, 443–450. [Google Scholar] [CrossRef]
- Bae, J.; Kang, S.; Lee, Y.; Jun, K. Synthesis of DME from syngas on the bifunctional Cu–ZnO–Al2O3/Zr-modified ferrierite: Effect of Zr content. Appl. Catal. B 2009, 90, 426–435. [Google Scholar] [CrossRef]
- Baltes, C.; Vukojević, S.; Schüth, F. Correlations between synthesis; precursor; and catalyst structure and activity of a large set of CuO/ZnO/Al2O3 catalysts for methanol synthesis. J. Catal. 2008, 258, 334–344. [Google Scholar] [CrossRef]









| Catalyst | Grain Size/nm | Textural Properties | ||
|---|---|---|---|---|
| 2θ ≈ 35.5° | 2θ ≈ 38.7° | Surface Area (m2/g) | Pore Volume (cm3/g) | |
| WWC | 18.8 | 14.4 | 43.9 | 0.27 |
| EWC | 12.0 | 13.9 | 56.4 | 0.31 |
| WMC | 8.9 | 11.2 | 59.5 | 0.29 |
| EMC | 8.4 | 9.5 | 77.2 | 0.36 |
| Catalyst | Surface Atom/% | BE(Cu 2p3/2) /eV | BE(Zn 2p3/2) /eV | KE(Cu LMM) /eV | KE(Zn LMM) /eV | ||
|---|---|---|---|---|---|---|---|
| Cu | Zn | XCu/XZn | |||||
| WWC | 6.56 | 15.61 | 0.42 | 932.00 | 1021.45 | 919.10 | 988.30 |
| EWC | 4.96 | 9.17 | 0.54 | 932.05 | 1020.60 | 919.05 | 988.35 |
| WMC | 5.49 | 8.7 | 0.63 | 932.10 | 1020.35 | 918.95 | 988.80 |
| EMC | 10.77 | 1.2 | 8.98 | 932.95 | 1020.15 | 918.20 | 988.85 |
| Catalyst | Solvent | Heating Manner a | XMeOH /% | STYH2 /mL·g−1·h−1 | SCO /% b |
|---|---|---|---|---|---|
| WWC | Water | WB | 53.6 | 300.0 | 1.53 |
| EWC | Ethanol | WB | 59.1 | 333.9 | 0.39 |
| WMC | Water | MI | 85.0 | 479.5 | 0.82 |
| EMC | Ethanol | MI | 91.2 | 516.7 | 0.29 |
| Catalyst | Precursor | Solvent | Heating Manner |
|---|---|---|---|
| WWC | WWP | Water | Water bath (WB) |
| EWC | EWP | Ethanol | Water bath (WB) |
| WMC | WMP | Water | Microwave irradiation (MI) |
| EMC | EMP | Ethanol | Microwave irradiation (MI) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Liu, Y.; Zhang, J. Hydrogen Production via Methanol Steam Reforming over CuO/ZnO/Al2O3 Catalysts Prepared via Oxalate-Precursor Synthesis. Catalysts 2023, 13, 1335. https://doi.org/10.3390/catal13101335
Wang H, Liu Y, Zhang J. Hydrogen Production via Methanol Steam Reforming over CuO/ZnO/Al2O3 Catalysts Prepared via Oxalate-Precursor Synthesis. Catalysts. 2023; 13(10):1335. https://doi.org/10.3390/catal13101335
Chicago/Turabian StyleWang, Haiguang, Yongfeng Liu, and Jun Zhang. 2023. "Hydrogen Production via Methanol Steam Reforming over CuO/ZnO/Al2O3 Catalysts Prepared via Oxalate-Precursor Synthesis" Catalysts 13, no. 10: 1335. https://doi.org/10.3390/catal13101335