Efficient and Stable O-Methylation of Catechol with Dimethyl Carbonate over Aluminophosphate Catalysts
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of APO Catalysts
2.2. O-Methylation of Catechol over APO Catalysts
3. Materials and Methods
3.1. Materials
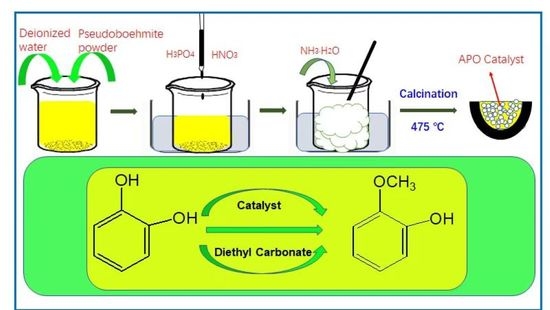
3.2. Catalyst Preparation
3.3. Catalyst Characterization
3.4. Catalytic Activity Tests
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ren, X.; Xu, D.; Yin, Y.; Zou, X.; Wang, Y.; Shang, X.; Wang, X. High catalytic performance and sustainability of Zr modified aluminophosphate for vapor-phase selective O-methylation of catechol with methanol. Catalysts 2021, 11, 740. [Google Scholar] [CrossRef]
- Kakhlon, O.; Ferreira, I.; Solmesky, L.; Weil, M.; Senderowitz, H.; Lossos, A.; Alvarez, R.; Pampou, S.; Escriba, P.; Yue, W. Guaiacol can be a drug-candidate for treating adult polyglucosan body disease. Neurology 2018, 17, 99694. [Google Scholar]
- Campos-Franzani, M.I.; Gajardo-Parra, N.F.; Pazo-Carballo, C.; Aravena, P.; Santiago, R.; Palomar, J.; Escalona, N.; Canales, R.I. Extraction of guaiacol from hydrocarbons as an alternative for the upgraded bio-oil purification: Experimental and computational thermodynamic study. Fuel 2020, 280, 118405. [Google Scholar] [CrossRef]
- Li, N.; Su, J.; Wang, H.; Cavaco-Paulo, A. Production of antimicrobial powders of guaiacol oligomers by a laccase-catalyzed synthesis reaction. Process Biochem. 2021, 111, 213–220. [Google Scholar] [CrossRef]
- Mao, H.; Li, X.; Xu, F.; Xiao, Z.; Zhang, W.; Meng, T. Vapour-phase selective O-methylation of catechol with methanol over metal phosphate catalysts. Catalysts 2021, 11, 531. [Google Scholar] [CrossRef]
- Jafari, A.A.; Khodadadi, A.; Mortazavi, Y. Vapor-phase selective o-alkylation of catechol with methanol over lanthanum phosphate and its modified catalysts with Ti and Cs. J. Mol. Catal. A Chem. 2013, 372, 79–83. [Google Scholar] [CrossRef]
- Chen, F.; Wei, W.; Gao, Y.; Wang, Y.; Yan, Z.; Zhang, Z.; Yu, H.; Xu, G.; Shi, L. Synthesis of highly effective [Emim]IM applied in one-step CO2 conversion to dimethyl carbonate. J. CO2 Util. 2022, 65, 102178. [Google Scholar] [CrossRef]
- Medrano-García, J.D.; Javaloyes-Antón, J.; Vázquez, D.; Ruiz-Femenia, R.; Caballero, J.A. Alternative carbon dioxide utilization in dimethyl carbonate synthesis and comparison with current technologies. J. CO2 Util. 2021, 45, 101436. [Google Scholar] [CrossRef]
- Liu, K.; Liu, C. One-pot synthesis of dimethyl carbonate over a binary catalyst of an ionic liquid and an alkali carbonate under low pressure. ACS Omega 2021, 6, 13839–13846. [Google Scholar] [CrossRef]
- Moulay, S. O-Methylation of hydroxyl-containing organic substrates: A comprehensive overview. Curr. Org. Chem. 2018, 22, 1986–2016. [Google Scholar] [CrossRef]
- Yadav, G.D.; Tekale, S.P. Selective O-alkylation of 2-naphthol using phosphonium-based ionic liquid as the phase transfer catalyst. Org. Process Res. Dev. 2010, 14, 722–727. [Google Scholar] [CrossRef]
- Talawar, M.B.; Jyoth, T.M.; Sawant, P.D.; Rajab, T.; Raob, B.S. Calcined Mg–Al hydrotalcite as an efficient catalyst for the synthesis of guaiacol. Green Chem. 2000, 2, 266–268. [Google Scholar] [CrossRef]
- Parida, K.; Das, J. Mg/Al hydrotalcites: Preparation, characterisation and ketonisation of acetic acid. J. Mol. Catal. A Chem. 2000, 151, 185–192. [Google Scholar] [CrossRef]
- Vijayaraj, M.; Gopinath, C.S. Selective production of methoxyphenols from dihydroxybenzenes on alkali metal ion-loaded MgO. J. Catal. 2006, 243, 376–388. [Google Scholar] [CrossRef]
- Luque, R.; Campelo, J.M.; Conesa, T.D.; Luna, D.; Marinasa, J.M.; Romero, A.A. Catechol O-methylation with dimethyl carbonate over different acid–base catalysts. New J. Chem. 2006, 30, 1228–1234. [Google Scholar] [CrossRef]
- Kabra, S.K.; Huuhtanen, M.; Keiski, R.L.; Yadav, G.D. Selectivity engineering of O-methylation of hydroxybenzenes with dimethyl carbonate using ionic liquid as catalyst. React. Chem. Eng. 2016, 1, 330–339. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, J.; Xie, G.; Yin, Z.; Liu, J.; Ma, X.B. Hollow, mesoporous, eutectic Zn1−xMgxO nano-spheres as solid acid–base catalysts for the highly regio-selective O-methylation of 1,2-diphenols. Catal. Sci. Technol. 2021, 11, 7454–7466. [Google Scholar] [CrossRef]
- Xu, D.; Ren, J.; Yue, S.; Zou, X.; Shang, X.; Wang, X. One-pot synthesis of Al-P-O catalysts and their catalytic properties for O-methylation of catechol and methanol. Materials 2021, 14, 5492. [Google Scholar] [CrossRef]
- Bang, Y.; Han, S.J.; Yoo, J.; Choi, J.H.; Lee, J.K.; Song, J.H.; Lee, J.; Song, I.K. Hydrogen production by steam reforming of simulated liquefied natural gas (LNG) over nickel catalyst supported on mesoporous phosphorus-modified alumina xerogel. Appl. Catal. B Environ. 2014, 148, 269–280. [Google Scholar] [CrossRef]
- Ratthiwal, J.; Lazaro, N.; Reyes, A.A.S.; Osman, S.M.; Reubroycharoen, P.; Luque, R. Batch and continuous-flow room temperature furfural acetalization with ethanol over aluminophosphate (APAl) catalysts for biofuels production. Fuel 2022, 332, 126049. [Google Scholar] [CrossRef]
- Dawaymeh, F.; Elmutasim, O.; Gaber, D.; Gaber, S.; Reddy, K.S.K.; Basina, G. Metal substitution effects of aluminophosphate AlPO4-5 as solid acid catalyst for esterification of acetic acid with ethanol. Mol. Catal. 2021, 501, 111371. [Google Scholar] [CrossRef]
- Tavan, Y. Coated HZSM-5 catalyst with aluminophosphate for methanol dehydration to produce dimethyl ether reaction. Asia-Pacific. J. Chem. Eng. 2014, 9, 449–455. [Google Scholar] [CrossRef]
- Prajapati, R.; Jadav, D.; Pandey, M.; Nishimura, K.; Inagaki, S.; Kubota, Y.; Bandyopadhyay, R.; Bandyopadhyay, M. Synthesis of hierarchical silicoaluminophosphate (SAPO) molecular seves by post-synthetic modification and their catalytic application. Eur. J. Inorg. Chem. 2022, 2022, e202200185. [Google Scholar] [CrossRef]
- Martinez-Franco, R.; Cantin, A.; Vidal-Moya, A.; Moliner, M.; Corma, A. Self-assembled aromatic molecules as efficient organic structure directing agents to synthesize the silicoaluminophosphate SAPO-42 with isolated Si species. Chem. Mater. A Publ. Am. Chem. Soc. 2015, 27, 2981–2989. [Google Scholar] [CrossRef]
- Yadav, R.; Singh, A.K.; Sakthivel, A. Unique mesoporous silicoaluminophosphate assembled from faujasite-type SAPO-37 precursor: A potential catalyst for isomerization. Chem. Lett. 2013, 42, 1160–1162. [Google Scholar] [CrossRef]
- Chen, Y.; Xiang, B.F.; Jiang, L.H.; Zhang, F.M.; Liu, C.B.; Wang, Y.M.; Zheng, Y.N.; Liu, Z.H.; He, P. Selective hydrogenation of α-pinene on a nickel supported aluminophosphate catalyst: Process optimization and reaction kinetics. Int. J. Chem. Kinet. 2020, 53, 440–456. [Google Scholar]
- Jin, Y.X.; Ke, Q.P.; Li, D.D.; Lei, Z.; Ling, Q.; Xu, J.; Cui, P. Nickel doped aluminophosphate-5 as an efficient heterogeneous catalyst for Imine synthesis by direct condensation of alcohols and amines at room temperature. Chem. Sel. 2018, 3, 2812–2818. [Google Scholar] [CrossRef]
- Vijayasankar, A.V.; Nagaraju, N. Preparation and characterisation of amorphous mesoporous aluminophosphate and metal aluminophosphate as an efficient heterogeneous catalyst for transesterification reaction. C. R. Chim. 2011, 14, 1109–1116. [Google Scholar] [CrossRef]
- de Souza, E.C.; Pereira, M.M.; Lam, Y.L.; Morgado, E.; Chinelatto, L.S. Aluminum phosphate as active matrix of fluid catalytic cracking catalysts: Y zeolite stabilization. Appl. Catalysis. A Gen. 2021, 619, 118156. [Google Scholar] [CrossRef]
- Ma, T.L.; Ding, J.F.; Liu, X.L.; Chen, G.L.; Zheng, J.D. Gas-phase dehydration of glycerol to acrolein over different metal phosphate catalysts. Korean J. Chem. Eng. 2020, 37, 955–960. [Google Scholar] [CrossRef]
- Blasco, T.; Corma, A.; Fernandez, L.; Fornes, V.; Guil-Lopez, R. Magic angle spinning NMR investigations on amorphous aluminophosphate oxynitrides. Phys. Chem. Chem. Phys. 1999, 1, 4493–4499. [Google Scholar] [CrossRef]
- Fang, W.; Riisager, A. Efficient valorization of biomass-derived furfural to fuel bio-additive over aluminum phosphate. Appl. Catal. B Environ. 2021, 298, 120575. [Google Scholar] [CrossRef]
- Wu, Z.; Li, Z.; Li, C. Cooperative catalytic effects between the penta-coordinated Al and Al2O3 in Al2O3-AlPO4 for aldol condensation of methyl acetate with formaldehyde to methyl acrylate. Chin. J. Chem. Eng. 2022, 52, 172–183. [Google Scholar] [CrossRef]
- Wang, X.; Lv, T.; Wu, W.; Sui, J.; Liu, Q.; Liu, H.; Huang, J.; Jia, L. Aluminum doped solid acid with suitable ratio of Brønsted and Lewis acid sites synthesized by electric-flocculation of phosphotungstic acid via hydrothermal treatment for producing 5-hydroxymethylfurfural from glucose. Appl. Catal. A Gen. 2019, 574, 87–96. [Google Scholar] [CrossRef]
- Lee, Y.J.; Kim, Y.M.; Viswanadham, N.; Jun, K.W.; Bae, J.W. Novel aluminophosphate (AlPO) bound ZSM-5 extrudates with improved catalytic properties for methanol to propylene (MTP) reaction. Appl. Catal. A Gen. 2010, 374, 18–25. [Google Scholar] [CrossRef]
- Chada, R.R.; Enumula, S.S.; Reddy, K.S.; Gudimella, M.D.; Kamaraju, S.R.R.; Burri, D.R. Direct and facile synthesis of LaPO4 containing SBA-15 catalyst for selective O-methylation of phenol to anisole in continuous process. Microporous Mesoporous Mater. 2020, 300, 110144. [Google Scholar] [CrossRef]
- Jiang, X.; Wang, F.; Cai, W.; Zhang, X. Trisodium citrate-assisted synthesis of highly water-dispersible and superparamagnetic mesoporous Fe3O4 hollow microspheres via solvothermal process. J. Alloys Compd. 2015, 636, 34–39. [Google Scholar] [CrossRef]
- Fang, W.; Riisager, A. Improved catalytic transfer hydrogenation of biomass-derived aldehydes with metal-loaded aluminum phosphate. ACS Sustain. Chem. Eng. 2022, 10, 1536–1543. [Google Scholar] [CrossRef]
- Tang, C.; Peng, J.; Li, X.; Zhai, Z.; Bai, W.; Jiang, N.; Gao, H.; Liao, Y. Efficient and selective conversion of lactic acid into acetaldehyde using a mesoporous aluminum phosphate catalyst. Green Chem. 2015, 17, 1159. [Google Scholar] [CrossRef]
- Hamza, A.; Nagaraju, N. Amorphous metal-aluminophosphate catalysts for aldol condensation of n-heptanal and benzaldehyde to jasminaldehyde. Chin. J. Catal. 2015, 36, 209–215. [Google Scholar] [CrossRef]
- Nikitina, M.A.; Ivanova, I.I. Conversion of 2,3-Butanediol over Phosphate Catalysts. Eur. Soc. J. Catal. 2016, 8, 1346–1353. [Google Scholar] [CrossRef]
- Wang, H.; Wang, Y.; Liu, W.; Cai, H.; Lv, J.; Liu, J. Amorphous magnesium substituted mesoporous aluminophosphate: An acid-base sites synergistic catalysis for transesterification of diethyl carbonate and dimethyl carbonate in fixed-bed reactor. Microporous Mesoporous Mater. 2020, 292, 109757. [Google Scholar] [CrossRef]
- Rawat, S.; Singh, B.; Kumar, R.; Pendem, C.; Bhandari, S.; Natte, K.; Narani, A. Value addition of lignin to zingerone using recyclable AlPO4 and Ni/LRC catalysts. Chem. Eng. J. 2022, 431, 134130. [Google Scholar] [CrossRef]
- Smolakova, L.; Frolich, K.; Troppova, I.; Kutalek, P.; Kroft, E.; Capek, L. Determination of base sites in Mg–Al mixed oxides by combination of TPD-CO2 and CO2 adsorption calorimetry. J. Anal. Calorim. 2016, 127, 1921–1929. [Google Scholar] [CrossRef]
- Wang, X.; Wang, R.; Gu, X.; Jia, J.; Zheng, Z. Light-assisted O-methylation of phenol with dimethyl carbonate over a layered double oxide catalyst. Catal. Sci. Technol. 2019, 9, 1774. [Google Scholar] [CrossRef]
- Jyothi, T.M.; Raja, T.; Talawar, M.B.; Rao, B.S. Selective O-methylation of catechol using dimethyl carbonate over calcined Mg-Al hydrotalcites. Appl. Catal. A Gen. 2001, 211, 41–46. [Google Scholar] [CrossRef]
- Baylon, R.A.L.; Sun, J.M.; Kovarik, L.; Engelhard, M.; Li, H.; Winkelman, A.D.; Wang, Y. Structural identification of ZnxZryOz catalysts for cascade aldolization and self-deoxygenation reactions. Appl. Catal. B Environ. 2018, 234, 337–346. [Google Scholar] [CrossRef]
- Mallesham, B.; Sudarsanam, P.; Reddy, B.M. Eco-friendly synthesis of bio-additive fuels from renewable glycerol using nanocrystalline SnO2-based solid acids. Catal. Sci. Technol. 2014, 4, 803. [Google Scholar] [CrossRef]
- Campelo, J.M.; Jaraba, M.; Luna, D.; Luque, R.; Marinas, J.M.; Romero, A.A. Effect of phosphate precursor and organic additives on the structural and catalytic properties of amorphous mesoporous AlPO4 materials. Chem. Mater. 2003, 15, 3352–3364. [Google Scholar] [CrossRef]
- Qian, G.; Xu, X.; Sun, W.; Xu, Y.; Liu, Q. Preparation, characterization, and stability of calcium zinc hydrophosphate. Mater. Res. Bull. 2008, 43, 3463–3473. [Google Scholar] [CrossRef]
- Li, F.; France, L.J.; Cai, Z.; Li, Y.; Liu, S.; Lou, H.; Long, J.; Li, X. Catalytic transfer hydrogenation of butyl levulinate to γ-valerolactone over zirconium phosphates with adjustable Lewis and Brønsted acid sites. Appl. Catal. B Environ. 2017, 214, 67–77. [Google Scholar] [CrossRef]







| Sample | Specific Surface Area (m2 g−1) a | P/Al Molar Ratio b |
|---|---|---|
| APO(0.6)-475 | 116 | 0.58 |
| APO(0.7)-475 | 120 | 0.66 |
| APO(0.8)-475 | 125 | 0.79 |
| APO(0.9)-475 | 35 | 0.85 |
| APO(0.7)-375 | 124 | 0.71 |
| APO(0.7)-425 | 123 | 0.68 |
| APO(0.7)-525 | 117 | 0.65 |
| APO(0.7)-575 | 116 | 0.63 |
| Catalyst | Temperature (°C) | Conversion (%) | Selectivity (%) | Ref |
|---|---|---|---|---|
| AlP1.1Zr0.012-400 | 280 | 82 | 89 | [1] |
| M-P-O | 270 | 80 | 92 | [5] |
| La1−xTixPO4 | 275 | 75 | 79 | [6] |
| Mg–Al | 325 | 94 | 79 | [12] |
| AlPO4–Al2O3 | 300 | 11.7 | 87 | [15] |
| Al-P-O | 275 | 58 | 97 | [18] |
| APO(0.7)-475 | 300 | 94 | 63 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, B.; Sheng, Y.; Zhou, L.; Hong, R.; Zhang, L.; Ren, X.; Zou, X.; Shang, X.; Lu, X.; Wang, X. Efficient and Stable O-Methylation of Catechol with Dimethyl Carbonate over Aluminophosphate Catalysts. Catalysts 2023, 13, 150. https://doi.org/10.3390/catal13010150
Wu B, Sheng Y, Zhou L, Hong R, Zhang L, Ren X, Zou X, Shang X, Lu X, Wang X. Efficient and Stable O-Methylation of Catechol with Dimethyl Carbonate over Aluminophosphate Catalysts. Catalysts. 2023; 13(1):150. https://doi.org/10.3390/catal13010150
Chicago/Turabian StyleWu, Baoqin, Yao Sheng, Linkai Zhou, Runduo Hong, Lifan Zhang, Xinfeng Ren, Xiujing Zou, Xingfu Shang, Xionggang Lu, and Xueguang Wang. 2023. "Efficient and Stable O-Methylation of Catechol with Dimethyl Carbonate over Aluminophosphate Catalysts" Catalysts 13, no. 1: 150. https://doi.org/10.3390/catal13010150