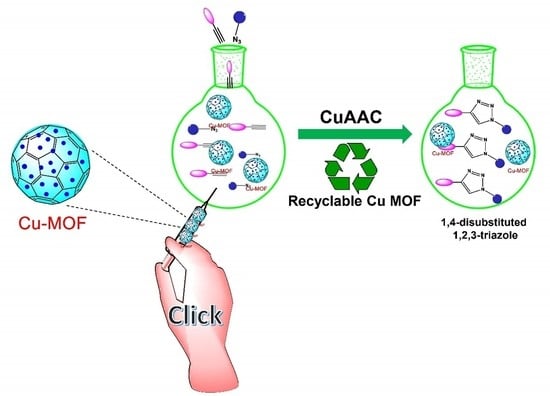
Copper-Based Metal–Organic Frameworks (MOFs) as an Emerging Catalytic Framework for Click Chemistry
Abstract
:1. Introduction
2. MOFs as Heterogenous Catalysts
3. Physico-Chemical Properties of MOFs
4. Methods for the Synthesis of MOFs
5. MOFs as Catalysts for Innumerable Reactions
6. Reactions Catalyzed by Cu-Tailored MOFs


7. Reaction Mechanism for CuAAC Using MOF Catalyst
8. Copper-Based MOFs as Click Catalysts for Spectacular CuAAC
9. Challenges in the Synthesis and Applications of Cu-Based MOFs
10. Conclusions and Future Aspects
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| MOF | Metal–organic framework |
| Im | Imidazolate |
| BTC | Benzene tricarboxylate |
| BDC | Benzene dicarboxylate |
| PyDC | Pyridine-3,5-dicarboxylate |
| TPP | Triphenylphosphonium |
| CPA | 4-Chloro-phenyl)-pyridin-4-ylmethylene-amine |
| Himdc | 4,5-Imidazoledicarboxylate |
| Bipy | 4,4′-Bipyridine |
| DABCO | 1,4-Diazabicyclo [2.2.2] octane |
| 2-pymo | 2-Hydroxypyrimidinolate |
| SBU | Supplementary building units |
| OCMC | O-Carboxymethyl chitosan |
| IM | Imidazolium |
| HPHT | High pressure and high temperature |
| MtbPtpB | Mycobacterium tuberculosis protein tyrosine phosphatase B |
References
- Kolb, H.C.; Finn, M.G.; Sharpless, K.B. Click Chemistry: Diverse Chemical Function from a Few Good Reactions. Angew. Chem. Int. Ed. 2001, 40, 2004–2021. [Google Scholar] [CrossRef]
- Hou, J.; Liu, X.; Shen, J.; Zhao, G.; Wang, P.G. The Impact of Click Chemistry in Medicinal Chemistry. Expert Opin. Drug Discov. 2012, 7, 489–501. [Google Scholar] [CrossRef] [PubMed]
- Kolb, H.C.; Sharpless, K.B. The Growing Impact of Click Chemistry on Drug Discovery. Drug Discov. Today 2003, 8, 1128–1137. [Google Scholar] [CrossRef]
- Singh, G.; George, N.; Singh, R.; Singh, G.; Sushma; Kaur, G.; Singh, H.; Singh, J. Ion Recognition by 1,2,3-Triazole Moieties Synthesized via “Click Chemistry”. Appl. Organomet. Chem. 2022, 37, e6897. [Google Scholar] [CrossRef]
- Ahmad Fuaad, A.A.H.; Azmi, F.; Skwarczynski, M.; Toth, I. Peptide Conjugation via CuAAC ‘Click’ Chemistry. Molecules 2013, 18, 13148–13174. [Google Scholar] [CrossRef] [Green Version]
- Hein, J.E.; Fokin, V.V. Copper-Catalyzed Azide–Alkyne Cycloaddition (CuAAC) and beyond: New Reactivity of Copper(I) Acetylides. Chem. Soc. Rev. 2010, 39, 1302–1315. [Google Scholar] [CrossRef]
- Kalra, P.; Kaur, R.; Singh, G.; Singh, H.; Singh, G.; Pawan; Kaur, G.; Singh, J. Metals as “Click” Catalysts for Alkyne-Azide Cycloaddition Reactions: An Overview. J. Organomet. Chem. 2021, 944, 121846. [Google Scholar] [CrossRef]
- Saini, P.; Sonika; Singh, G.; Kaur, G.; Singh, J.; Singh, H. Robust and Versatile Cu(I) Metal Frameworks as Potential Catalysts for Azide-Alkyne Cycloaddition Reactions: Review. Mol. Catal. 2021, 504, 111432. [Google Scholar] [CrossRef]
- Kaboudin, B.; Mostafalu, R.; Yokomatsu, T. Fe3O4 Nanoparticle-Supported Cu(II)-β-Cyclodextrin Complex as a Magnetically Recoverable and Reusable Catalyst for the Synthesis of Symmetrical Biaryls and 1,2,3-Triazoles from Aryl Boronic Acids. Green Chem. 2013, 15, 2266–2274. [Google Scholar] [CrossRef]
- Zhang, W.; He, X.; Ren, B.; Jiang, Y.; Hu, Z. Cu(OAc)2·H2O—An efficient catalyst for Huisgen-click reaction in supercritical carbon dioxide. Tetrahedron Lett. 2015, 56, 2472–2475. [Google Scholar] [CrossRef]
- Zirak, M.; Garegeshlagi, E.J. Picolinimidoamide-Cu(II) complex anchored on Fe3O4@SiO2 core–shell magnetic nanoparticles: An efficient reusable catalyst for click reaction. J. Coord. Chem. 2018, 71, 1168–1179. [Google Scholar] [CrossRef]
- Khojastehnezhad, A.; Bakavoli, M.; Javid, A.; Khakzad Siuki, M.M.; Shahidzadeh, M. Synthesis, characterization, and investigation of catalytic activity of copper(II) porphyrin graphene oxide for azide–alkyne cycloaddition. Res. Chem. Intermed. 2019, 45, 4473–4485. [Google Scholar] [CrossRef]
- Kaur, P.; Lal, B.; Kaur, N.; Singh, G.; Singh, A.; Kaur, G.; Singh, J. Selective Two Way Cd(II) and Co(II) Ions Detection by 1,2,3–Triazole Linked Fluorescein Derivative. J. Photochem. Photobiol. Chem. 2019, 382, 111847. [Google Scholar] [CrossRef]
- Deilam, R.; Moeinpour, F.; Mohseni-Shahri, F.S. Catalytic performance of Cu(II)-supported graphene quantum dots modified NiFe2O4 as a proficient nano-catalyst in the synthesis of 1,2,3-triazoles. Mon. Chem. 2020, 151, 1153–1162. [Google Scholar] [CrossRef]
- Jin, T.; Yan, M.; Yamamoto, Y. Click Chemistry of Alkyne-Azide Cycloaddition using Nanostructured Copper Catalysts. ChemCatChem 2012, 4, 1217–1229. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, Y.; Huang, Q.; Lin, X.; Zeb, A.; Wu, Y.; Xu, Z.; Xu, X. Recent Advances in Cu-Based Metal–Organic Frameworks and Their Derivatives for Battery Applications. ACS Appl. Energy Mater. 2022, 5, 7842–7873. [Google Scholar] [CrossRef]
- Singh, N.; Thakur, A. Applications of Copper Based Metal Organic Frameworks. Mater. Today Proc. 2022, 50, 1906–1911. [Google Scholar] [CrossRef]
- Li, L.; Zou, J.; Han, Y.; Liao, Z.; Lu, P.; Nezamzadeh-Ejhieh, A.; Liu, J.; Peng, Y. Recent Advances in Al(III)/In(III)-Based MOFs for the Detection of Pollutants. New J. Chem. 2022, 46, 19577–19592. [Google Scholar] [CrossRef]
- Dong, X.; Li, Y.; Li, D.; Liao, D.; Qin, T.; Prakash, O.; Kumar, A.; Liu, J. A New 3D 8-Connected Cd(II) MOF as a Potent Photocatalyst for Oxytetracycline Antibiotic Degradation. CrystEngComm 2022, 24, 6933–6943. [Google Scholar] [CrossRef]
- Guo, M.; Zhang, M.; Liu, R.; Zhang, X.; Li, G. State-of-the-Art Advancements in Photocatalytic Hydrogenation: Reaction Mechanism and Recent Progress in Metal-Organic Framework (MOF)-Based Catalysts. Adv. Sci. 2022, 9, 2103361. [Google Scholar] [CrossRef]
- Lawson, H.D.; Walton, S.P.; Chan, C. Metal–Organic Frameworks for Drug Delivery: A Design Perspective. ACS Appl. Mater. Interfaces 2021, 13, 7004–7020. [Google Scholar] [CrossRef]
- Qin, L.; Liang, F.; Li, Y.; Wu, J.; Guan, S.; Wu, M.; Xie, S.; Luo, M.; Ma, D. A 2D Porous Zinc-Organic Framework Platform for Loading of 5-Fluorouracil. Inorganics 2022, 10, 202. [Google Scholar] [CrossRef]
- Butler, K.S.; Pearce, C.J.; Nail, E.A.; Vincent, G.A.; Gallis, D.F.S. Antibody Targeted Metal–Organic Frameworks for Bioimaging Applications. ACS Appl. Mater. Interfaces 2020, 12, 31217–31224. [Google Scholar] [CrossRef]
- Song, Z.; Zhang, L.; Doyle-Davis, K.; Fu, X.; Luo, J.-L.; Sun, X. Recent Advances in MOF-Derived Single Atom Catalysts for Electrochemical Applications. Adv. Energy Mater. 2020, 10, 2001561. [Google Scholar] [CrossRef]
- Zhang, W.; Ye, G.; Liao, D.; Chen, X.; Lu, C.; Nezamzadeh-Ejhieh, A.; Khan, M.S.; Liu, J.; Pan, Y.; Dai, Z. Recent Advances of Silver-Based Coordination Polymers on Antibacterial Applications. Molecules 2022, 27, 7166. [Google Scholar] [CrossRef]
- Baumann, A.E.; Burns, D.A.; Liu, B.; Thoi, V.S. Metal-Organic Framework Functionalization and Design Strategies for Advanced Electrochemical Energy Storage Devices. Commun. Chem. 2019, 2, 86. [Google Scholar] [CrossRef] [Green Version]
- Sharmin, E.; Zafar, F.; Sharmin, E.; Zafar, F. Introductory Chapter: Metal-organic Frameworks (MOFs); IntechOpen: London, UK, 2016; ISBN 978-953-51-2663-8. [Google Scholar] [CrossRef] [Green Version]
- Kirchon, A.; Feng, L.; Drake, H.F.; Joseph, E.A.; Zhou, H.-C. From fundamentals to applications: A toolbox for robust and multifunctional MOF materials. Chem. Soc. Rev. 2018, 47, 8611. [Google Scholar] [CrossRef] [PubMed]
- Li, P.-Z.; Wang, X.-J.; Zhao, Y. Click chemistry as a versatile reaction for construction and modification of metal-organic frameworks. Coord. Chem. Rev. 2019, 380, 484–518. [Google Scholar] [CrossRef]
- Luz, I.; i Xamena, F.X.L.; Corma, A. Bridging Homogeneous and Heterogeneous Catalysis with MOFs: “Click” Reactions with Cu-MOF Catalysts. J. Catal. 2010, 276, 134–140. [Google Scholar] [CrossRef]
- Zhou, H.-C.; Long, J.R.; Yaghi, O.M. Introduction to Metal–Organic Frameworks. Chem. Rev. 2012, 112, 673–674. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.-S.; Zhang, M.; Zou, R.; Xu, Q. Metal–Organic Framework-Based Catalysts with Single Metal Sites. Chem. Rev. 2020, 120, 12089–12174. [Google Scholar] [CrossRef] [PubMed]
- Doonan, C.J.; Sumby, C.J. Metal–Organic Framework Catalysis. CrystEngComm 2017, 19, 4044–4048. [Google Scholar] [CrossRef]
- Li, S.; Gao, Y.; Li, N.; Ge, L.; Bu, X.; Feng, P. Transition Metal-Based Bimetallic MOFs and MOF-Derived Catalysts for Electrochemical Oxygen Evolution Reaction. Energy Environ. Sci. 2021, 14, 1897–1927. [Google Scholar] [CrossRef]
- Kumar, S.; Mohan, B.; Tao, Z.; You, H.; Ren, P. Incorporation of Homogeneous Organometallic Catalysts into Metal–Organic Frameworks for Advanced Heterogenization: A Review. Catal. Sci. Technol. 2021, 11, 5734–5771. [Google Scholar] [CrossRef]
- Wang, Q.; Astruc, D. State of the Art and Prospects in Metal–Organic Framework (MOF)-Based and MOF-Derived Nanocatalysis. Chem. Rev. 2020, 120, 1438–1511. [Google Scholar] [CrossRef]
- Ali, M.; Pervaiz, E.; Noor, T.; Rabi, O.; Zahra, R.; Yang, M. Recent advancements in MOF- based catalysts for applications in electrochemical and photoelectrochemical water splitting: A review. Int. J. Energy Res. 2020, 45, 1190–1226. [Google Scholar] [CrossRef]
- Chassaing, S.; Bénéteau, V.; Pale, P. When CuAAC “Click Chemistry” Goes Heterogeneous. Catal. Sci. Technol. 2016, 6, 923–957. [Google Scholar] [CrossRef]
- Chen, B.W.J.; Xu, L.; Mavrikakis, M. Computational Methods in Heterogeneous Catalysis. Chem. Rev. 2021, 121, 1007–1048. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Zhou, T. Computational Design of Heterogeneous Catalysts and Gas Separation Materials for Advanced Chemical Processing. Front. Chem. Sci. Eng. 2021, 15, 49–59. [Google Scholar] [CrossRef]
- Vojvodic, A.; Nørskov, J.K. New design paradigm for heterogeneous catalysts. Natl. Sci. Rev. 2015, 2, 140–149. [Google Scholar] [CrossRef] [Green Version]
- Kang, Y.-S.; Lu, Y.; Chen, K.; Zhao, Y.; Wang, P.; Sun, W.-Y. Metal–organic frameworks with catalytic centers: From synthesis to catalytic application. Coord. Chem. Rev. 2019, 378, 262–280. [Google Scholar] [CrossRef]
- Butova, V.V.; Soldatov, M.A.; Guda, A.A.; Lomachenko, K.A.; Lamberti, C. Metal-Organic Frameworks: Structure, Properties, Methods of Synthesis and Characterization. Russ. Chem. Rev. 2016, 85, 280–307. [Google Scholar] [CrossRef]
- Redfern, L.R.; Farha, O.K. Mechanical Properties of Metal–Organic Frameworks. Chem. Sci. 2019, 10, 10666–10679. [Google Scholar] [CrossRef] [Green Version]
- Moosavi, S.M.; Nandy, A.; Jablonka, K.M.; Ongari, D.; Janet, J.P.; Boyd, P.G.; Lee, Y.; Smit, B.; Kulik, H.J. Understanding the Diversity of the Metal-Organic Framework Ecosystem. Nat. Commun. 2020, 11, 4068. [Google Scholar] [CrossRef]
- Kabtamu, D.M.; Wu, Y.; Li, F. Hierarchically Porous Metal–Organic Frameworks: Synthesis Strategies, Structure(s), and Emerging Applications in Decontamination. J. Hazard. Mater. 2020, 397, 122765. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Yan, D. Long-Afterglow Metal–Organic Frameworks: Reversible Guest-Induced Phosphorescence Tunability. Chem. Sci. 2016, 7, 4519–4526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuang, X.; Ma, Y.; Su, H.; Zhang, J.; Dong, Y.-B.; Tang, B. High-Performance Liquid Chromatographic Enantioseparation of Racemic Drugs Based on Homochiral Metal–Organic Framework. Anal. Chem. 2014, 86, 1277–1281. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Wang, J.; Zhang, W.; Yang, C.; Zhang, L.; Zhu, W.; Sun, J.; Li, G.; Li, T.; Wang, J. Amorphous Fe/Mn Bimetal–Organic Frameworks: Outer and Inner Structural Designs for Efficient Arsenic(III) Removal. J. Mater. Chem. A 2019, 7, 2845–2854. [Google Scholar] [CrossRef]
- McKinstry, C.; Cussen, E.J.; Fletcher, A.J.; Patwardhan, S.V.; Sefcik, J. Scalable Continuous Production of High Quality HKUST-1 via Conventional and Microwave Heating. Chem. Eng. J. 2017, 326, 570–577. [Google Scholar] [CrossRef]
- Kholdeeva, O.A.; Skobelev, I.Y.; Ivanchikova, I.D.; Kovalenko, K.A.; Fedin, V.P.; Sorokin, A.B. Hydrocarbon Oxidation over Fe- and Cr-Containing Metal-Organic Frameworks MIL-100 and MIL-101–a Comparative Study. Catal. Today 2014, 238, 54–61. [Google Scholar] [CrossRef]
- Chen, Y.; Xiao, J.; Lv, D.; Huang, T.; Xu, F.; Sun, X.; Xi, H.; Xia, Q.; Li, Z. Highly Efficient Mechanochemical Synthesis of an Indium Based Metal-Organic Framework with Excellent Water Stability. Chem. Eng. Sci. 2017, 158, 539–544. [Google Scholar] [CrossRef]
- Yang, H.; Orefuwa, S.; Goudy, A. Study of Mechanochemical Synthesis in the Formation of the Metal–Organic Framework Cu3(BTC)2 for Hydrogen Storage. Microporous Mesoporous Mater. 2011, 143, 37–45. [Google Scholar] [CrossRef]
- Li, Y.-P.; Zhang, L.-J.; Ji, W.-J. Synthesis, Characterization, Crystal Structure of Magnesium Compound Based 3, 3′, 5, 5′-Azobenzentetracarboxylic Acid and Application as High-Performance Heterogeneous Catalyst for Cyanosilylation. J. Mol. Struct. 2017, 1133, 607–614. [Google Scholar] [CrossRef]
- Du, J.; Zou, G. A Novel Microporous Zinc(II) Metal-Organic Framework with Highly Selectivity Adsorption of CO2 over CH4. Inorg. Chem. Commun. 2016, 69, 20–23. [Google Scholar] [CrossRef]
- Lestari, W.W.; Arvinawati, M.; Martien, R.; Kusumaningsih, T. Green and Facile Synthesis of MOF and Nano MOF Containing Zinc(II) and Benzen 1,3,5-Tri Carboxylate and Its Study in Ibuprofen Slow-Release. Mater. Chem. Phys. 2018, 204, 141–146. [Google Scholar] [CrossRef]
- Czaja, A.U.; Trukhan, N.; Müller, U. Industrial applications of metal–organic frameworks. Chem. Soc. Rev. 2009, 38, 1284–1293. [Google Scholar] [CrossRef]
- Dey, C.; Kundu, T.; Biswal, B.P.; Mallick, A.; Banerjee, R. Crystalline Metal-Organic Frameworks (MOFs): Synthesis, Structure and Function. Acta Crystallogr. Sect. B Struct. Sci. Cryst. Eng. Mater. 2014, 70, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.T.L.; Nguyen, C.V.; Dang, G.H.; Le, K.K.A.; Phan, N.T.S. Towards Applications of Metal–Organic Frameworks in Catalysis: Friedel–Crafts Acylation Reaction over IRMOF-8 as an Efficient Heterogeneous Catalyst. J. Mol. Catal. Chem. 2011, 349, 28–35. [Google Scholar] [CrossRef]
- Naskar, K.; Maity, S.; Maity, H.S.; Sinha, C. A Reusable Efficient Green Catalyst of 2D Cu-MOF for the Click and Knoevenagel Reaction. Molecules 2021, 26, 5296. [Google Scholar] [CrossRef]
- Kikhtyanin, O.; Kubička, D.; Čejka, J. Toward Understanding of the Role of Lewis Acidity in Aldol Condensation of Acetone and Furfural Using MOF and Zeolite Catalysts. Catal. Today 2015, 243, 158–162. [Google Scholar] [CrossRef]
- Zhao, T.; Dong, M.; Yang, L.; Liu, Y. Synthesis of Stable Hierarchical MIL-101(Cr) with Enhanced Catalytic Activity in the Oxidation of Indene. Catalysts 2018, 8, 394. [Google Scholar] [CrossRef] [Green Version]
- Rostamnia, S.; Alamgholiloo, H.; Liu, X. Pd-grafted Open Metal Site Copper-benzene-1,4-dicarboxylate Metal Organic Frameworks (Cu-BDC MOF’s) as Promising Interfacial Catalysts for Sustainable Suzuki Coupling. J. Colloid Interface Sci. 2016, 469, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Tai, X.; Zhou, X. Au3+/Au0 Supported on Chromium(III) Terephthalate Metal Organic Framework (MIL-101) as an Efficient Heterogeneous Catalystfor Three-Component Coupling Synthesis of Propargylamines. Materials 2017, 10, 99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hajiashrafi, T.; Karimi, M.; Heydari, A.; Tehrani, A.A. Erbium-Organic Framework as Heterogeneous Lewis Acid Catalysis for Hantzsch Coupling and Tetrahydro-4H-Chromene Synthesis. Catal. Lett. 2017, 147, 453–462. [Google Scholar] [CrossRef]
- Phan, N.T.S.; Le, K.K.A.; Phan, T.D. MOF-5 as an Efficient Heterogeneous Catalyst for Friedel–Crafts Alkylation Reactions. Appl. Catal. Gen. 2010, 2, 246–253. [Google Scholar] [CrossRef]
- Gascon, J.; Aktay, U.; Hernandez Alonso, M.; van Klink, G.; Kapteijn, F. Amino-Based Metal Organic Frameworks as Stable, Highly Active Basic Catalysts. J. Catal. 2009, 261, 75–87. [Google Scholar] [CrossRef]
- Torbina, V.; Salaev, M.; Vodyankina, O. Effect of Solvent Nature on Propylene Glycol Oxidation with Tert-Butyl Hydroperoxide over Metal–Organic Framework Cr-MIL-101. RSC Adv. 2019, 9, 25981–25986. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phan, N.T.S.; Nguyen, T.T.; Nguyen, C.V.; Nguyen, T.T. Ullmann-Type Coupling Reaction Using Metal-Organic Framework MOF-199 as an Efficient Recyclable Solid Catalyst. Appl. Catal. Gen. 2013, 457, 69–77. [Google Scholar] [CrossRef]
- Ullah, L.; Zhao, G.; Xu, Z.; He, H.; Usman, M.; Zhang, S. 12-Tungstophosphoric Acid Niched in Zr-Based Metal-Organic Framework: A Stable and Efficient Catalyst for Friedel-Crafts Acylation. Sci. China Chem. 2018, 61, 402–411. [Google Scholar] [CrossRef]
- Calleja, G.; Sanz, R.; Orcajo, G.; Briones, D.; Leo, P.; Martínez, F. Copper-based MOF-74 material as effective acid catalyst in Friedel–Crafts acylation of anisole. Catal. Today 2014, 227, 130–137. [Google Scholar] [CrossRef]
- Zhu, C.; Mao, Q.; Li, D.; Li, C.; Zhou, Y.; Wu, X.; Luo, Y.; Li, Y. A Readily Available Urea Based MOF That Act as a Highly Active Heterogeneous Catalyst for Friedel-Crafts Reaction of Indoles and Nitrostryenes. Catal. Commun. 2018, 104, 123–127. [Google Scholar] [CrossRef]
- Zhu, Y.; Chen, J.; Kaskel, S. Porphyrin-Based Metal-Organic Frameworks for Biomedical Applications. Angew. Chem. Int. Ed. 2020, 60, 5010–5035. [Google Scholar] [CrossRef] [Green Version]
- Dong, W.; Zhang, L.; Wang, C.; Feng, C.; Shang, N.; Gao, S.; Wang, C. Palladium Nanoparticle Embedded in Metal Organic Framework Derived Porous Carbon: Synthesis and Application for Efficient Suzuki-Miyaura Coupling Reaction. RSC Adv. 2016, 6, 37118–37123. [Google Scholar] [CrossRef]
- Hartmann, M.; Fischer, M. Amino-functionalized basic catalysts with MIL-101 structure. Microporous Mesoporous Mater. 2012, 164, 38–43. [Google Scholar] [CrossRef]
- Arefi, E.; Khojastehnezhad, A.; Shiri, A. A Magnetic Copper Organic Framework Material as an Efficient and Recyclable Catalyst for the Synthesis of 1,2,3-Triazole Derivatives. Sci. Rep. 2021, 11, 20514. [Google Scholar] [CrossRef]
- Konnerth, H.; Matsagar, B.M.; Chen, S.S.; Prechtl, M.H.G.; Shieh, F.-K.; Wu, K.C.-W. Metal-Organic Framework (MOF)-Derived Catalysts for Fine Chemical Production. Coord. Chem. Rev. 2020, 416, 213319. [Google Scholar] [CrossRef]
- Luz, I.; i Xamena, F.L.; Corma, A. Bridging Homogeneous and Heterogeneous Catalysis with MOFs: Cu-MOFs as Solid Catalysts for Three-Component Coupling and Cyclization Reactions for the Synthesis of Propargylamines, Indoles and Imidazopyridines. J. Catal. 2012, 285, 285–291. [Google Scholar] [CrossRef]
- Kal-Koshvandi, A.T.; Maleki, A.; Tarlani, A.; Soroush, M.R. Synthesis and Characterization of Ultrapure HKUST-1 MOFs as Reusable Heterogeneous Catalysts for the Green Synthesis of Tetrazole Derivatives. ChemistrySelect 2020, 5, 3164–3172. [Google Scholar] [CrossRef]
- Mollabagher, H.; Taheri, S.; Mojtahedi, M.M.; Seyedmousavi, S. Cu-Metal Organic Frameworks (Cu-MOF) as an Environment-Friendly and Economical Catalyst for One Pot Synthesis of Tacrine Derivatives. RSC Adv. 2020, 10, 1995–2003. [Google Scholar] [CrossRef]
- Ghaffarian, F.; Ghasemzadeh, M.; Aghaei, S. An efficient synthesis of some new curcumin based pyrano[2,3-d]pyrimidine-2,4(3H)-dione derivatives using CoFe2O4@OCMC@Cu(BDC) as a novel and recoverable catalyst. J. Mol. Struct. 2019, 1186, 204–211. [Google Scholar] [CrossRef]
- Ghasemzadeh, M.A.; Ghaffarian, F. Preparation of Core/Shell/Shell CoFe2O4/OCMC/Cu (BDC) Nanostructure as a Magnetically Heterogeneous Catalyst for the Synthesis of Substituted Xanthenes, Quinazolines and Acridines under Ultrasonic Irradiation. Appl. Organomet. Chem. 2020, 34, e5580. [Google Scholar] [CrossRef]
- Barea, E.; Navarro, J.A.R.; Salas, J.M.; Masciocchi, N.; Galli, S.; Sironi, A. Mineralomimetic Sodalite- and Muscovite-Type Coordination Frameworks. Dynamic Crystal-to-Crystal Interconversion Processes Sensitive to Ion Pair Recognition. J. Am. Chem. Soc. 2004, 126, 3014–3015. [Google Scholar] [CrossRef]
- Meldal, M.; Diness, F. Recent Fascinating Aspects of the CuAAC Click Reaction. Trends Chem. 2020, 2, 569–584. [Google Scholar] [CrossRef]
- Basu, P.; Bhanja, P.; Salam, N.; Dey, T.K.; Bhaumik, A.; Das, D.; Islam, S.M. Silver Nanoparticles Supported over Al2O3@Fe2O3 Core-Shell Nanoparticles as an Efficient Catalyst for One-Pot Synthesis of 1,2,3-Triazoles and Acylation of Benzyl Alcohol. Mol. Catal. 2017, 439, 31–40. [Google Scholar] [CrossRef]
- Willig, J.C.M.; Granetto, G.; Reginato, D.; Dutra, F.R.; Poruczinski, F.; de Oliveira, I.M.; Stefani, H.A.; de Campos, S.D.; de Campos, A.; Manarin, F.; et al. A Comparative Study between Cu(INA)2-MOF and [Cu(INA)2(H2O)4] Complex for a Click Reaction and the Biginelli Reaction under Solvent-Free Conditions. RSC Adv. 2020, 10, 3407–3415. [Google Scholar] [CrossRef] [Green Version]
- Tourani, H.; Naimi-Jamal, M.R.; Panahi, L.; Dekamin, M.G. Nanoporous Metal-Organic Framework Cu2(BDC)2(DABCO) as an Efficient Heterogeneous Catalyst for One-Pot Facile Synthesis of 1,2,3-Triazole Derivatives in Ethanol: Evaluating Antimicrobial Activity of the Novel Derivatives. Sci. Iran. 2019, 26, 1485–1496. [Google Scholar] [CrossRef] [Green Version]
- Singh, G.; Mangat, S.S.; Sharma, H.; Singh, J.; Arora, A.; Singh Pannu, A.P.; Singh, N. Design and Syntheses of Novel Fluorescent Organosilicon-Based Chemosensors through Click Silylation: Detection of Biogenic Amines. RSC Adv. 2014, 4, 36834–36844. [Google Scholar] [CrossRef]
- Velpuri, V.R.; Muralidharan, K. Multicomponent Click Reaction Catalyzed by Organic Surfactant-Free Copper Sulfide (Sf-CuS) Nano/Micro Flowers. J. Organomet. Chem. 2019, 884, 59–65. [Google Scholar] [CrossRef]
- Beghdadi, S.; Miladi, I.A.; Addis, D.; Romdhane, H.B.; Bernard, J.; Drockenmuller, E. Synthesis and Polymerization of C-Vinyl- and N-Vinyl-1,2,3-Triazoles. Polym. Chem. 2012, 3, 1680–1692. [Google Scholar] [CrossRef]
- Ju, C.; Meng, C.; Ma, J.; Zhang, X.; Ding, S. Construction of Sequence-Defined Polytriazoles by IrAAC and CuAAC Reactions. Chem. Commun. 2020, 56, 3955–3958. [Google Scholar] [CrossRef]
- Chinchilla, R.; Nájera, C. Recent Advances in Sonogashira Reactions. Chem. Soc. Rev. 2011, 40, 5084–5121. [Google Scholar] [CrossRef] [PubMed]
- Bozorov, K.; Zhao, J.; Aisa, H.A. 1,2,3-Triazole-Containing Hybrids as Leads in Medicinal Chemistry: A Recent Overview. Bioorg. Med. Chem. 2019, 27, 3511–3531. [Google Scholar] [CrossRef] [PubMed]
- Shil, A.K.; Kumar, S.; Sharma, S.; Chaudhary, A.; Das, P. Polystyrene Resin Supported Palladium(0) (Pd@PR) Nanocomposite Mediated Regioselective Synthesis of 4-Aryl-1-Alkyl/(2-Haloalkyl)-1H-1,2,3-Triazoles and Their N-Vinyl Triazole Derivatives from Terminal Alkynes. RSC Adv. 2015, 5, 11506–11514. [Google Scholar] [CrossRef]
- Meldal, M.; Tornøe, C.W. Cu-Catalyzed Azide−Alkyne Cycloaddition. Chem. Rev. 2008, 108, 2952–3015. [Google Scholar] [CrossRef]
- Guo, X.; Lin, C.; Zhang, M.; Duan, X.; Dong, X.; Sun, D.; Pan, J.; You, T. 2D/3D Copper-Based Metal-Organic Frameworks for Electrochemical Detection of Hydrogen Peroxide. Front. Chem. 2021, 9, 743637. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, R.B.; Sandra E., D.; Harry, H. Experimental Evidence for the Involvement of Dinuclear Alkynylcopper(I) Complexes in Alkyne–Azide Chemistry. Chem. Eur. J. 2010, 16, 6278–6284. [Google Scholar] [CrossRef]
- Worrell, B.T.; Malik, J.A.; Fokin, V.V. Direct Evidence of a Dinuclear Copper Intermediate in Cu(I)-Catalyzed Azide-Alkyne Cycloadditions. Science 2013, 340, 457–460. [Google Scholar] [CrossRef] [Green Version]
- Mo, J.-N.; Su, J.; Zhao, J. The Asymmetric A3(Aldehyde–Alkyne–Amine) Coupling: Highly Enantioselective Access to Propargylamines. Molecules 2019, 24, 1216. [Google Scholar] [CrossRef] [Green Version]
- Swami, S.; Sahu, S.N.; Shrivastava, R. Nanomaterial Catalyzed Green Synthesis of Tetrazoles and Its Derivatives: A Review on Recent Advancements. RSC Adv. 2021, 11, 39058–39086. [Google Scholar] [CrossRef]
- He, Z.; Zhao, X.; Pan, X.; Li, Y.; Wang, X.; Xu, H.; Xu, Z. Ligand Geometry Controlling Zn-MOF Partial Structures for Their Catalytic Performance in Knoevenagel Condensation. RSC Adv. 2019, 9, 25170–25176. [Google Scholar] [CrossRef] [Green Version]
- LiLi, P.; Regati, S.; Huang, H.; Arman, H.D.; Zhao, J.C.-G.; Chen, B. A Metal–Organic Framework as a Highly Efficient and Reusable Catalyst for the Solvent-Free 1,3-Dipolar Cycloaddition of Organic Azides to Alkynes. Inorg. Chem. Front. 2015, 2, 42–46. [Google Scholar] [CrossRef]
- Fan, Z.; Wang, Z.; Cokoja, M.; Fischer, R.A. Defect Engineering: An Effective Tool for Enhancing the Catalytic Performance of Copper-MOFs for the Click Reaction and the A 3 Coupling. Catal. Sci. Technol. 2021, 11, 2396–2402. [Google Scholar] [CrossRef]
- Xu, Z.; Han, L.; Zhuang, G.; Bai, J.; Sun, D. In Situ Construction of Three Anion-Dependent Cu(I) Coordination Networks as Promising Heterogeneous Catalysts for Azide–Alkyne “Click” Reactions. Inorg. Chem. 2015, 54, 4737–4743. [Google Scholar] [CrossRef]
- Murugan, R.; Reddy, M.B.; Pandurangan, P.; Anandhan, R. Gold-like Thiolate-Protected Ultrasmall Cubic Copper Nanocluster-Based Metal–Organic Framework as a Selective Catalyst for Stepwise Synthesis of Unsymmetric Bistriazole by Click Reaction. ACS Appl. Mater. Interfaces 2020, 12, 56004–56016. [Google Scholar] [CrossRef]
- Hu, Y.-H.; Wang, J.-C.; Yang, S.; Li, Y.-A.; Dong, Y.-B. CuI@UiO-67-IM: A MOF-Based Bifunctional Composite Triphase-Transfer Catalyst for Sequential One-Pot Azide–Alkyne Cycloaddition in Water. Inorg. Chem. 2017, 56, 8341–8347. [Google Scholar] [CrossRef]
- Arnanz, A.; Pintado-Sierra, M.; Corma, A.; Iglesias, M.; Sanchez, F. Bifunctional Metal Organic Framework Catalysts for Multistep Reactions: MOF-Cu(BTC)-[Pd] Catalyst for One-Pot Heteroannulation of Acetylenic Compounds. Adv. Synth. Catal. 2012, 354, 1347–1355. [Google Scholar] [CrossRef]
- Jayaramulu, K.; Suresh, V.M.; Maji, T.K. Stabilization of Cu2O Nanoparticles on a 2D Metal–Organic Framework for Catalytic Huisgen 1,3-Dipolar Cycloaddition Reaction. Dalton Trans. 2014, 44, 83–86. [Google Scholar] [CrossRef]
- Jiang, W.; Yang, J.; Liu, Y.-Y.; Ma, J.-F. Porphyrin-Based Mixed-Valent Ag(I)/Ag(II) and Cu(I)/Cu(II) Networks as Efficient Heterogeneous Catalysts for the Azide–Alkyne “Click” Reaction and Promising Oxidation of Ethylbenzene. Chem. Commun. 2016, 52, 1373–1376. [Google Scholar] [CrossRef]
- Kim, A.; Muthuchamy, N.; Yoon, C.; Joo, S.H.; Park, K.H. MOF-Derived Cu@Cu2O Nanocatalyst for Oxygen Reduction Reaction and Cycloaddition Reaction. Nanomaterials 2018, 8, 138. [Google Scholar] [CrossRef]
- Thanh, N.D.; Hai, D.S.; Bich, V.T.N.; Hien, P.T.T.; Duyen, N.T.K.; Mai, N.T.; Dung, T.T.; Toan, V.N.; Van, H.T.K.; Dang, L.H.; et al. Efficient Click Chemistry towards Novel 1H-1,2,3-Triazole-Tethered 4H-Chromene−d-Glucose Conjugates: Design, Synthesis and Evaluation of in Vitro Antibacterial, MRSA and Antifungal Activities. Eur. J. Med. Chem. 2019, 167, 454–471. [Google Scholar] [CrossRef]
- Bikas, R.; Ajormal, F.; Noshiranzadeh, N.; Emami, M.; Kozakiewicz, A. 1D Azido Bridged Cu(II) Coordination Polymer with 1,3-oxazolidine Ligand as an Effective Catalyst for Green Click Synthesis of 1,2,3-triazoles. Appl. Organomet. Chem. 2020, 34. [Google Scholar] [CrossRef]
- Lu, B.-B.; Yang, J.; Che, G.-B.; Pei, W.-Y.; Ma, J.-F. Highly Stable Copper(I)-Based Metal–Organic Framework Assembled with Resorcin[4]Arene and Polyoxometalate for Efficient Heterogeneous Catalysis of Azide–Alkyne “Click” Reaction. ACS Appl. Mater. Interfaces 2018, 10, 2628–2636. [Google Scholar] [CrossRef] [PubMed]
- Tourani, H.; Naimi-Jamal, M.R.; Dekamin, M.G. Preparation of 5-Substituted-1H-Tetrazoles Catalyzed by MOFs via Two Strategies: Direct Condensation of Aryl Nitriles with SodiumAzide, and Tri-Component Reaction Method. ChemistrySelect 2018, 3, 8332–8337. [Google Scholar] [CrossRef]
- Jia, X.; Xu, G.; Du, Z.; Fu, Y. Cu(BTC)-MOF Catalyzed Multicomponent Reaction to Construct 1,4-Disubstituted-1,2,3-Triazoles. Polyhedron 2018, 151, 515–519. [Google Scholar] [CrossRef]
- Wang, Z.; Zhou, X.; Gong, S.; Xie, J. MOF-Derived Cu@N-C Catalyst for 1,3-Dipolar Cycloaddition Reaction. Nanomaterials 2022, 12, 1070. [Google Scholar] [CrossRef] [PubMed]
- Maity, T.; Saha, D.; Koner, S. Aromatic N-Arylations Catalyzed by Copper-Anchored Porous Zinc-Based Metal-Organic Framework under Heterogeneous Conditions. ChemCatChem 2014, 6, 2373–2383. [Google Scholar] [CrossRef]
- Wang, X.-X.; Yang, J.; Xu, X.; Ma, J.-F. Highly Stable Copper(I)–Thiacalix[4]Arene-Based Frameworks for Highly Efficient Catalysis of Click Reactions in Water. Chem.-Eur. J. 2019, 25, 16660–16667. [Google Scholar] [CrossRef]
- Yamane, I.; Sato, K.; Otomo, R.; Yanase, T.; Miura, A.; Nagahama, T.; Kamiya, Y.; Shimada, T. Ultrahigh-Pressure Preparation and Catalytic Activity of MOF-Derived Cu Nanoparticles. Nanomaterials 2021, 11, 1040. [Google Scholar] [CrossRef]
- Fu, Q.; Xie, K.; Tan, S.; Ren, J.M.; Zhao, Q.; Webley, P.A.; Qiao, G.G. The Use of Reduced Copper Metal–Organic Frameworks to Facilitate CuAAC Click Chemistry. Chem. Commun. 2016, 52, 12226–12229. [Google Scholar] [CrossRef]
- Thanh, N.D.; Hai, D.S.; Ha, N.T.T.; Tung, D.T.; Le, C.T.; Van, H.T.K.; Toan, V.N.; Toan, D.N.; Dang, L.H. Synthesis, Biological Evaluation and Molecular Docking Study of 1,2,3-1H-Triazoles Having 4H-Pyrano[2,3-d]Pyrimidine as Potential Mycobacterium Tuberculosis Protein Tyrosine Phosphatase B Inhibitors. Bioorg. Med. Chem. Lett. 2019, 29, 164–171. [Google Scholar] [CrossRef]
- Shinde, K.S.; Michael, P.; Fuhrmann, D.; Binder, W.H. A Mechanochemically Active Metal-Organic Framework (MOF) Based on Cu-Bis-NHC-Linkers: Synthesis and Mechano-Catalytic Activation. Macromol. Chem. Phys. 2022, 223, 2200207. [Google Scholar] [CrossRef]
- De, D.; Pal, T.K.; Neogi, S.; Senthilkumar, S.; Das, D.; Gupta, S.S.; Bharadwaj, P.K. A Versatile CuII Metal–Organic Framework Exhibiting High Gas Storage Capacity with Selectivity for CO2: Conversion of CO2 to Cyclic Carbonate and Other Catalytic Abilities. Chem.-Eur. J. 2016, 22, 3387–3396. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zhang, Y.; Liu, Z.; Du, Z.; Zhang, L.; Ren, J.; Qu, X. A Biocompatible Heterogeneous MOF–Cu Catalyst for In Vivo Drug Synthesis in Targeted Subcellular Organelles. Angew. Chem. Int. Ed. 2019, 58, 6987–6992. [Google Scholar] [CrossRef]
- Zeng, F.; Pan, Y.; Luan, X.; Gao, Y.; Yang, J.; Wang, Y.; Song, Y. Copper Metal-Organic Framework Incorporated Mesoporous Silica as a Bioorthogonal Biosensor for Detection of Glutathione. Sens. Actuators B Chem. 2021, 345, 130382. [Google Scholar] [CrossRef]




















| Entry | Property | Description |
|---|---|---|
| 1. | Surface area |
|
| 2. | Porosity  |
|
| 3. | Structure tunability and Luminescence |
|
| 4. | Chirality |
|
| 5. | Crystallinity |
|
| Entry | Method | Description | Examples | References |
|---|---|---|---|---|
| 1. | Electrochemical synthesis | Electrochemical synthesis involves the addition of metal ions into a reaction mixture consisting of organic linkers and electrolytes via anodic dissolution. The significant benefit of this technique is that the anions often found with metals in salts may be eliminated, resulting in very pure compounds, since less time and energy are needed for reactions to take place under more benign circumstances. | ZIF-8, HKUST-1, MIL-100 (Fe). | [51] |
| 2. | Microwave-assisted synthesis | The use of microwaves to irradiate the reaction mixture results in the creation of nanoscale MOF crystals. There are several benefits to this synthesis, including high efficiency, shorter reaction times, phase selectivity, morphological control, and particle size reduction. | [Cu3(btc)2(H2O)3], HKUST-1 | [50] |
| 3. | Mechanochemical synthesis | Mechanical forces are introduced to complete the chemical reaction. Mechanochemical synthesis has the benefit of not requiring the use of organic solvents, which may be carcinogenic, poisonous, and damaging to the environment. Metal oxides are often employed as precursors in this procedure rather than metal salts. | InOF-1, Cu3(BTC)2-MOF | [52,53] |
| 4. | Slow evaporation method | This conventional method for MOF preparation involves the use of suitable solvents to dissolve the precursor materials and later on slow evaporation of the solvent at an adequate temperature. The synthesis of MOFs using this approach is hindered by the insolubility of the chemicals. Accordingly, a combination of solvents may be utilised to improve solubility. | [Cu(2,3-pydc)(bpp)]·2. 5H2O, [Zn(2,3-pydc)(bpp)]·2. 5H2O | [50] |
| 5. | Solvothermal synthesis | The reaction between the organic linker and metal ion takes place in a suitable solvent at a temperature above the boiling point of the solvent used. The primary benefit of this technique is that the relatively greater yield can be obtained. | Zn2(bpabdc)2(DMF)2(H2O)n, [Cu(tdc) (H2O)]n.n(DMA) | [54,55] |
| 6. | Sonochemical synthesis | The solution mixture was subjected to ultrasonic radiations (20 kHz–10 MHz) to synthesize MOF with novel morphologies. The sonochemical technique allows for the rapid production of MOFs with a tiny crystal size in a very short reaction time. This approach has the benefits of being fast, cheap, repeatable, and eco-friendly. | HKUST-1, TMU-7, [Zn3(btc)2] | [56] |
| Entry | Reaction Catalyzed | MOF Type | Metal in MOF | Reference |
|---|---|---|---|---|
| 1. | Friedel–Crafts reaction | HPW@Zr-BTC | Zirconium | [70] |
| Cu-MOF-74 | Copper | [71] | ||
| Urea containing 2D MOF | Copper | [72] | ||
| 4. | Suzuki coupling | Cu-BDC MOF | Copper | [63] |
| 5. | Heck coupling | Pd(II)-porphyrinic MOF | Palladium | [73] |
| 6. | Suzuki–Miyaura coupling | NPC-Pd MOF | Palladium | [74] |
| 7. | Knoevenagel condensation | Al-MIL-101-NH2 | Aluminium | [75] |
| 8. | Aldol condensation | Basolite F300 | Iron | [61] |
| 9. | CuAAC reaction | Fe3O4@HKUST-1 | Copper | [76] |
| Entry | Catalyst | Metal | Reaction Conditions | Solvent | Recyclability | References |
|---|---|---|---|---|---|---|
| 1. | Cu(INA)2-MOF | Cu(I) | 1.5 h, 80 °C | solvent free | 5 times | [86] |
| 2. | [Cu(CPA)(BDC)]n | Cu(I) | 7 h, 90 °C | H2O-MeOH (1:4) | 4 times | [60] |
| 3. | Cu2(BDC)2(DABCO) | Cu(I) | 45 min, 60 °C | ethanol | 5 times | [87] |
| 4. | CuI | Cu(I) | rt | no solvent | NA | [84] |
| 5. | CuBr(PPh3)3] | Cu(I) | 60 °C, 5 h | THF/TEA | NA | [88] |
| 6. | sf-CuS | Cu(I) | rt, 30 min | H2O | NA | [89] |
| 7. | AgN(CN)2 | Ag(I) | rt | DIPEAH 2O/ethylene glycol | NA | [90] |
| 8. | ZnEt2 | Zn(II) | rt, 18 h | THF | NA | [91] |
| 9. | Au(RD32) | Au(III) | 60 °C, H2O | EtN3 | NA | [92] |
| 10. | RuH2(CO)(PPh3)3 | Ru(II) | 80 °C, 2 h | PTC, H2O | NA | [93] |
| 11. | Pd@PR | Pd(0) | 8–12 h, 100 ℃ | DMF | NA | [94] |
| Entry | MOF | Catalytic Amount (mol %) | Reaction Conditions | Solvent | Yield of Triazole | Recovery of Catalyst % | Catalytic Runs | References |
|---|---|---|---|---|---|---|---|---|
| 1. | Cu-MOF | 5 mol % | 7 h, rt | CH2Cl2 | 94 | 87 | 5 | [102] |
| 2. | CuBTC–PyDC | 5 mol % | 3 h, 70 ℃ | Ethanol | - | 76 | 4 | [103] |
| 3. | {[Cu6(bpz)6(CH3CN)3(CN)3Br]· 2OH· 14CH3CN}n | 10 mol % | 2 h, 60 ℃ | DMF and DMSO solution | 99 | 70 | 6 | [104] |
| 4. | Cu-MOF | 5 mol % | 12 h, rt | EtOH/ H2O (2:1) | 95 | 88 | 5 | [105] |
| 5. | CuI@UiO-67-IM | 2 mol % | 80 °C, 2 h | Water, under air atmosphere | 90 | 5 | [106] | |
| 6. | MOF-Cu (BTC)-[Pd] | 2 mol % | 2 h, 50 °C | DMF | 100 | 85 | 2 | [107] |
| 7. | Cu2O@{[Zn(Himdc)(bipy)0.5]· DMF} MOF | 0.5 mol % | 7 h, 50 °C | t-BuOH–H2O (2:1), Et3N | 98 | - | 3 | [108] |
| 8. | Cu(INA)2-MOF | 1 mol % | 1.5 h, 80 °C | Solvent free | 98 | 90 | 5 | [86] |
| 9. | [Cu(I)6I6(Cu(II)-TPPP)].2DMF | 1 mol % | 12 h, 50 °C | Methanol and water (4:1) | 100 | 90 | 5 | [109] |
| 10. | Fe3O4@HKUST-1 | 1.8 mol % | 2 h, reflux | H2O | 92 | 87 | 5 | [76] |
| 11. | Cu@Cu2O | 2.3 mol % | 5 h, 50 °C | Water, t-BuOH (2:1) | 100 | - | - | [110] |
| 12. | Cu@MOF-5 | 2 mol % | 30 min, 79–80 °C | Ethanol | 98 | - | - | [111] |
| 13. | [Cu(CPA)(BDC)]n | 10 mol % | 7 h, 90 °C | H2O-MeOH (1:4) | 93 | 70 | 4 | [60] |
| Entry | MOF | Catalytic Amount (mol %) | Reaction Conditions | Solvent | Yield of Triazole | Recovery of Catalyst % | Catalytic Runs | References |
|---|---|---|---|---|---|---|---|---|
| 1. | [CuI4(SiW12O40)(L)]·6H2O· 2DMF | 30 mg | 12 h, 80 ℃ | Methanol | 99 | 99 | 5 | [114] |
| 2. | [Cu4Cl4L]·CH3OH·1.5H2O | 10 mg | 8 h, 60 °C | Water | 99 | - | 5 | [113] |
| 3. | Cu(BTC)-MOF | 10 mg | 16 h, RT | CH3OH | 91 | 87 | 3 | [115] |
| 4. | Cu2(BDC)2(DABCO) | 20 mg | 45 min, 60 °C | Ethanol | 98 | 92 | 5 | [87] |
| 5. | Cu@N-C(600) | 5 mg | 12 h, 50 °C | t-BuOH/H2O (3/1) | 98 | 93 | 4 | [116] |
| 6. | [Cu(H3L)(μ1,3-N3)(N3)]n | 4 mg | 3.5 h, 40 °C | Water | 93 | - | - | [112] |
| 7. | IRMOF-3-PI-Cu | 2 mg | 20 h, 90°C | DMSO | 90 | 80 | 5 | [117] |
| 8. | [Cu4Cl4L]·CH3OH·1.5H2O | 10 mg | 8 h, 60 °C | Water | 99 | 97 | 5 | [118] |
| 9. | Cu-BTC | 10 mg | 1 h, 60 °C | 1,4-Dioxane, TEA | - | - | - | [119] |
| 10. | rCu-MOF | 0.2 mg | 4 h, 60 °C | H2O, N2 for 20 min | - | - | 3 | [120] |
| Entry | MOF | Catalytic Amount (mol %) | Reaction Conditions | Solvent | Yield of Triazole % | Catalytic Runs | References |
|---|---|---|---|---|---|---|---|
| 1. | Cu-Bis-NHC- MOF | 10 wt % | - | THF/MeOH | - | - | [122] |
| 2. | {[Cu2(L)(H2O)2]·(5 DMF)(4 H2O)}n | 5 wt % | 4 h, 50 °C | CH2Cl2 | 96 | - | [123] |
| 3. | [Cu(2-pymo)2] | - | 4 h, 70 °C | Ethanol | - | 6 | [30] |
| 4. | MOF-Cu-TPP | - | - | - | - | 10 | [124] |
| 5. | MSNs@Cu- MOF | - | - | - | - | - | [125] |
| 6. | Cu@MOF-5 | - | 4–5 h, 79−80 °C | abs. EtOH | 94 | - | [121] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, R.; Singh, G.; George, N.; Singh, G.; Gupta, S.; Singh, H.; Kaur, G.; Singh, J. Copper-Based Metal–Organic Frameworks (MOFs) as an Emerging Catalytic Framework for Click Chemistry. Catalysts 2023, 13, 130. https://doi.org/10.3390/catal13010130
Singh R, Singh G, George N, Singh G, Gupta S, Singh H, Kaur G, Singh J. Copper-Based Metal–Organic Frameworks (MOFs) as an Emerging Catalytic Framework for Click Chemistry. Catalysts. 2023; 13(1):130. https://doi.org/10.3390/catal13010130
Chicago/Turabian StyleSingh, Riddima, Gurleen Singh, Nancy George, Gurjaspreet Singh, Sofia Gupta, Harminder Singh, Gurpreet Kaur, and Jandeep Singh. 2023. "Copper-Based Metal–Organic Frameworks (MOFs) as an Emerging Catalytic Framework for Click Chemistry" Catalysts 13, no. 1: 130. https://doi.org/10.3390/catal13010130