Enriching SO42− Immobilization on α-Fe2O3 via Spatial Confinement for Robust NH3-SCR Denitration
Abstract
:1. Introduction
2. Results and Discussion
2.1. The Encapsulation of Fe2O3 in the Mesopores (TEM and N2 Sorption)
2.2. Surface Dispersion Properties of Fe2O3 in the Fe2O3/SBA-15 and Fe2O3/SiO2 Catalysts (XRD and XPS)
2.3. Effects of the Mesopore Confinement on the Capture of SO2 on Fe2O3
2.4. Effects of Enriched Sulfate on the Acid and Redox Properties of the Catalysts (NH3-TPD, NO Oxidation)
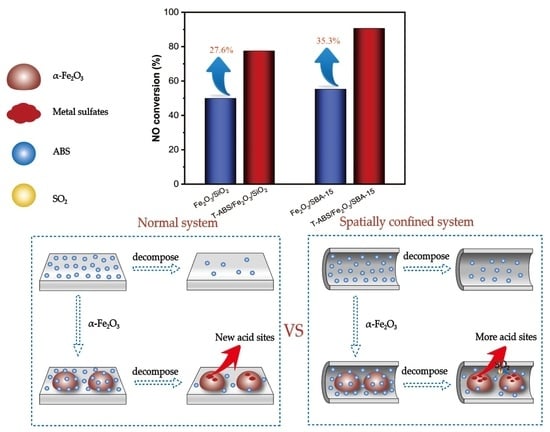
2.5. Promotion Effect of the SO42− Immobilization on the Catalytic Performance of α-Fe2O3 in NH3-SCR
3. Experimental Section
3.1. Catalysts Preparation
3.2. Catalyst Characterization
3.3. Activity Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bendrich, M.; Scheuer, A.; Hayes, R.; Votsmeier, M. Unified mechanistic model for Standard SCR, Fast SCR, and NO2 SCR over a copper chabazite catalyst. Appl. Catal. B Environ. 2018, 222, 76–87. [Google Scholar] [CrossRef]
- Busca, G.; Lietti, L.; Ramis, G.; Berti, F. Chemical and mechanistic aspects of the selective catalytic reduction of NOx by ammonia over oxide catalysts: A review. Appl. Catal. B Environ. 1998, 18, 1–36. [Google Scholar] [CrossRef]
- Alemany, L.J.; Lietti, L.; Ferlazzo, N.; Forzatti, P.; Busca, G.; Giamello, E.; Bregani, F. Reactivity and Physicochemical Characterization of V2O5-WO3/TiO2 De-NOx Catalysts. J. Catal. 1995, 155, 117–130. [Google Scholar] [CrossRef]
- Li, H.; Yi, X.; Miao, J.; Chen, Y.; Chen, J.; Wang, J. Improved Sulfur Resistance of COMMERCIAl V2O5-WO3/TiO2 SCR Catalyst Modified by Ce and Cu. Catalysts 2021, 11, 906. [Google Scholar] [CrossRef]
- Chen, L.; Si, Z.; Wu, X.; Weng, D.; Ran, R.; Yu, J. Rare earth containing catalysts for selective catalytic reduction of NOx with ammonia: A Review. J. Rare Earths 2014, 32, 907–917. [Google Scholar] [CrossRef]
- Xie, R.; Ma, L.; Li, Z.; Qu, Z.; Yan, N.; Li, J. Review of Sulfur Promotion Effects on Metal Oxide Catalysts for NOx Emission Control. ACS Catal. 2021, 11, 13119–13139. [Google Scholar] [CrossRef]
- Chen, Y.; Li, C.; Chen, J.; Tang, X. Self-Prevention of Well-Defined-Facet Fe2O3/MoO3 against Deposition of Ammonium Bisulfate in Low-Temperature NH3–SCR. Environ. Sci. Technol. 2018, 52, 11796–11802. [Google Scholar] [CrossRef]
- Qi, X.; Han, L.; Deng, J.; Lan, T.; Wang, F.; Shi, L.; Zhang, D. SO2-Tolerant Catalytic Reduction of NOx via Tailoring Electron Transfer between Surface Iron Sulfate and Subsurface Ceria. Environ. Sci. Technol. 2022, 56, 5840–5848. [Google Scholar] [CrossRef]
- Yu, Y.; Tan, W.; An, D.; Wang, X.; Liu, A.; Zou, W.; Tang, C.; Ge, C.; Tong, Q.; Sun, J.; et al. Insight into the SO2 resistance mechanism on γ-Fe2O3 catalyst in NH3-SCR reaction: A collaborated experimental and DFT study. Appl. Catal. B Environ. 2020, 281, 119544. [Google Scholar] [CrossRef]
- Tang, C.; Wang, H.; Dong, S.; Zhuang, J.; Qu, Z. Study of SO2 effect on selective catalytic reduction of NO with NH3 over Fe/CNTs: The change of reaction route. Catal. Today 2018, 307, 2–11. [Google Scholar] [CrossRef]
- Feng, C.; Han, L.; Wang, P.; Liu, X.; Zhou, G.; Zhang, D. Unraveling SO2-tolerant mechanism over Fe2(SO4)3/TiO2 catalysts for NO reduction. J. Environ. Sci. 2021, 111, 340–350. [Google Scholar] [CrossRef] [PubMed]
- Ni, J.; Peng, X.; Yang, L.; Zhang, K.; Zhang, Y.; Zhou, Y.; Wang, X.; Au, C.-T.; Jiang, L. Effects of cerium and tungsten addition on acid-base properties of spindle-like α-Fe2O3 in low-temperature SCR of NO with NH3. J. Rare Earths 2022, in press. [Google Scholar] [CrossRef]
- Wang, H.; Qu, Z.; Dong, S.; Xie, H.-B.; Tang, C. Superior Performance of Fe1–xWxOδ for the Selective Catalytic Reduction of NOx with NH3: Interaction between Fe and W. Environ. Sci. Technol. 2016, 50, 13511–13519. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-M.; Ma, Y.-P.; Chen, X.-Y.; Xu, S.-Y.; Chen, J.-D.; Zhang, Q.-L.; Zhao, B.; Ning, P. Promoting effect of SO2−4 functionalization on the performance of Fe2O3 catalyst in the selective catalytic reduction of NO with NH3. J. Fuel Chem. Technol. 2020, 48, 584–593. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, S.; Zhu, X.; Xu, X.; Huang, F.; Yang, Z.; Sun, C. Effects of sulfation on hematite for selective catalytic reduction of nitrogen oxides with ammonia. J. Colloid Interface Sci. 2021, 606, 1445–1456. [Google Scholar] [CrossRef]
- Guo, K.; Zhu, Y.; Yan, Z.; Liu, A.; Du, X.; Wang, X.; Tan, W.; Li, L.; Sun, J.; Tong, Q.; et al. The dual effects of ammonium bisulfate on the selective catalytic reduction of NO with NH3 over Fe2O3-WO3 catalyst confined in MCM-41. Chem. Eng. J. 2020, 389, 124271. [Google Scholar] [CrossRef]
- Dai, J.-P.; Li, D.; He, Y.-L.; Du, S.; Li, J.-N. Pore-scale investigation on the multi-component gas adsorption and diffusion in carbon xerogel microporous structure using molecular simulation methods. Microporous Mesoporous Mater. 2022, 337, 111890. [Google Scholar] [CrossRef]
- Ma, K.; Zou, W.; Zhang, L.; Li, L.; Yu, S.; Tang, C.; Gao, F.; Dong, L. Construction of hybrid multi-shell hollow structured CeO2–MnOx materials for selective catalytic reduction of NO with NH3. RSC Adv. 2017, 7, 5989–5999. [Google Scholar] [CrossRef]
- Ravikovitch, P.I.; Neimark, A.V. Experimental Confirmation of Different Mechanisms of Evaporation from Ink-Bottle Type Pores: Equilibrium, Pore Blocking, and Cavitation. Langmuir 2002, 18, 9830–9837. [Google Scholar] [CrossRef]
- Sietsma, J.R.; Friedrich, H.; Broersma, A.; Versluijs-Helder, M.; Van Dillen, A.J.; De Jongh, P.E.; De Jong, K.P. How nitric oxide affects the decomposition of supported nickel nitrate to arrive at highly dispersed catalysts. J. Catal. 2008, 260, 227–235. [Google Scholar] [CrossRef] [Green Version]
- Janssen, A.H.; Yang, C.-M.; Wang, Y.; Schüth, F.; Koster, A.J.; de Jong, K.P. Localization of Small Metal (Oxide) Particles in SBA-15 Using Bright-Field Electron Tomography. J. Phys. Chem. B 2003, 107, 10552–10556. [Google Scholar] [CrossRef]
- Guo, K.; Ji, J.; Osuga, R.; Zhu, Y.; Sun, J.; Tang, C.; Kondo, J.N.; Dong, L. Construction of Fe2O3 loaded and mesopore confined thin-layer titania catalyst for efficient NH3-SCR of NOx with enhanced H2O/SO2 tolerance. Appl. Catal. B Environ. 2021, 287, 119982. [Google Scholar] [CrossRef]
- Vaschetto, E.G.; Rodríguez, P.A.O.; Casuscelli, S.G.; Elías, V.R.; Eimer, G.A. Treatment of glyphosate contaminated wastewater based on the development of FE doped SBA-15 as advanced catalysts for wet oxidation process under room conditions. Catal. Today 2022, 394–396, 143–149. [Google Scholar] [CrossRef]
- Mahmoudabadi, Z.S.; Rashidi, A.; Maklavany, D.M. Optimizing treatment of alcohol vinasse using a combination of advanced oxidation with porous α-Fe2O3 nanoparticles and coagulation-flocculation. Ecotoxicol. Environ. Saf. 2022, 234, 113354. [Google Scholar] [CrossRef] [PubMed]
- Bukhtiyarova, G.; Deliy, I.; Sakaeva, N.; Kaichev, V.; Plyasova, L.; Bukhtiyarov, V. Effect of the calcination temperature on the properties of Fe2O3/SiO2 catalysts for oxidation of hydrogen sulfide. React. Kinet. Catal. Lett. 2007, 92, 89–97. [Google Scholar] [CrossRef]
- Wang, J.; Shao, X.; Zhang, Q.; Ma, J.; Ge, H. Preparation and photocatalytic application of magnetic Fe2O3/SBA-15 nanomaterials. J. Mol. Liq. 2018, 260, 304–312. [Google Scholar] [CrossRef]
- Ma, K.; Guo, K.; Li, L.; Zou, W.; Tang, C.; Dong, L. Cavity size dependent SO2 resistance for NH3-SCR of hollow structured CeO2-TiO2 catalysts. Catal. Commun. 2019, 128, 105719. [Google Scholar] [CrossRef]
- Guo, K.; Fan, G.; Gu, D.; Yu, S.; Ma, K.; Liu, A.; Tan, W.; Wang, J.; Du, X.; Zou, W.; et al. Pore Size Expansion Accelerates Ammonium Bisulfate Decomposition for Improved Sulfur Resistance in Low-Temperature NH3-SCR. ACS Appl. Mater. Interfaces 2019, 11, 4900–4907. [Google Scholar] [CrossRef]
- Tan, W.; Wang, J.; Li, L.; Liu, A.; Song, G.; Guo, K.; Luo, Y.; Liu, F.; Gao, F.; Dong, L. Gas phase sulfation of ceria-zirconia solid solutions for generating highly efficient and SO2 resistant NH3-SCR catalysts for NO removal. J. Hazard. Mater. 2019, 388, 121729. [Google Scholar] [CrossRef]
- Ma, L.; Li, J.; Ke, R.; Fu, L. Catalytic Performance, Characterization, and Mechanism Study of Fe2(SO4)3/TiO2 Catalyst for Selective Catalytic Reduction of NOx by Ammonia. J. Phys. Chem. C 2011, 115, 7603–7612. [Google Scholar] [CrossRef]
- García-Bordejé, E.; Pinilla, J.; Lázaro, M.; Moliner, R. NH3-SCR of NO at low temperatures over sulphated vanadia on carbon-coated monoliths: Effect of H2O and SO2 traces in the gas feed. Appl. Catal. B Environ. 2006, 66, 281–287. [Google Scholar] [CrossRef]
- Zhao, D.; Huo, Q.; Feng, J.; Chmelka, B.F.; Stucky, G.D. Nonionic Triblock and Star Diblock Copolymer and Oligomeric Surfactant Syntheses of Highly Ordered, Hydrothermally Stable, Mesoporous Silica Structures. J. Am. Chem. Soc. 1998, 120, 6024–6036. [Google Scholar] [CrossRef]







| Samples | Detected SO42− Concentration (mg/L) | Theoretical Maximum Concentration (mg/L) | Extra SO42− Concentration Derived from Fe2O3 Trapping (mg/L) |
|---|---|---|---|
| ABS/SBA-15 (untreated) | 38.58 | 39.71 | - |
| T-ABS/SBA-15 * | 18.49 | 39.71 | 7.84 |
| T-ABS/Fe2O3/SBA-15 | 26.33 | 39.71 | |
| ABS/SiO2 (untreated) | 39.03 | 39.71 | - |
| T-ABS/SiO2 | 9.80 | 39.71 | 3.04 |
| T-ABS/Fe2O3/SiO2 | 12.85 | 39.71 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, Z.; Cheng, L.; Tan, C.; Sin, S.; Huang, C.; Tang, C. Enriching SO42− Immobilization on α-Fe2O3 via Spatial Confinement for Robust NH3-SCR Denitration. Catalysts 2022, 12, 991. https://doi.org/10.3390/catal12090991
Gu Z, Cheng L, Tan C, Sin S, Huang C, Tang C. Enriching SO42− Immobilization on α-Fe2O3 via Spatial Confinement for Robust NH3-SCR Denitration. Catalysts. 2022; 12(9):991. https://doi.org/10.3390/catal12090991
Chicago/Turabian StyleGu, Zhiwen, Lijun Cheng, Chong Tan, Songil Sin, Chunkai Huang, and Changjin Tang. 2022. "Enriching SO42− Immobilization on α-Fe2O3 via Spatial Confinement for Robust NH3-SCR Denitration" Catalysts 12, no. 9: 991. https://doi.org/10.3390/catal12090991