2.1. Surface Morphology of Pt–Pd–Rh–Ru Gauzes Used in the Oxidation of NH3
Figure 1 displays SEM images of a fragment of the Pt–Pd–Rh–Ru gauze and wire surface after NH
3 oxidation in an industrial reactor at ca. 1173 K and ca. 7.0 bar for 3000 h. One can see that the gauze is covered with a continuous etched layer of porous crystal agglomerates (“cauliflowers”) with the size of ca. 25–50 µm (
Figure 1a,b). The “cauliflowers” have a porous structure with a pore sizes of 0.1–2.0 µm (
Figure 1c,d). Porous crystal structures of different shape and size are detected between “cauliflowers” (
Figure 1b,c). Images in
Figure 1 indicate a strong catalytic etching initiated by the oxidation of NH
3. Such “cauliflowers” were also detected on the surface of Pt–Rh gauzes after the oxidation of NH
3 in industrial reactors [
8,
9,
10].
Figure 2 shows SEM images of typical regions of the wire surface on the fresh Pt–Pd–Rh–Ru gauze (a), as well as on the back (b) and frontal (c) sides of the first gauze along the gas flow after its operation in NH
3 oxidation at 1133 K for 50 h in a laboratory reactor. Images of the indicated gauze regions were presented in our earlier study [
21]. To show the etching degree of the wire surface layer, a smooth surface of the wire, on which the etched structures (protrusions, crystal fragments and “cauliflowers”) reside, is marked in red (
Figure 2a–c). For the unused gauze, mostly the smooth surface regions are observed (
Figure 2a), whereas on both sides of the spent gauze there are crystal structures of different shapes and sizes. The back side of the gauze has a non-uniform etched layer with smooth regions and various crystals (
Figure 2b). The frontal side of the gauze has a continuous etched layer of “cauliflowers” (
Figure 2c), as on such gauze after its industrial operation (
Figure 1a,b).
In our studies [
18,
19,
20,
21], SEM and X-ray diffraction (XRD) were used to investigate the morphology and microstructure of the Pt–Pd–Rh–Ru gauze with the same composition as in this study after treatment at
T = 1133 K for 50 h in air or ammonia, and also after the oxidation of NH
3 with air. It was shown that the treatment medium produced a considerable effect on the morphology and microstructure of the gauze surface. The new gauze had mostly a smooth surface (
Figure 2a) and its specific surface area was equal to 26.0 cm
2·g
−1. After treatment of the gauzes in NH
3 and in air, grains with smooth and microfaceted surface were detected [
18], while after NH
3 oxidation, deep etching of the gauze surface was observed [
19,
20,
21]. After the oxidation of NH
3, on the back side of the first gauze along the gas flow we observed a non-uniform etched layer with the specific surface area of 52 cm
2·g
−1, which includes regions 100–200 µm in size with a smooth surface and with ca. 3 µm crystals [
19]. In
Figure 2b, such a region is represented by the smooth regions marked in red (left part of the image) and by the regions with protrusions and crystals (central and right parts of the image), respectively. On the frontal side of the spent gauze, there was a continuous etched layer of porous “cauliflowers” with a size of ca. 10 µm and specific surface area of 260 cm
2·g
−1 (
Figure 2c) [
20]. For the fresh gauze and both sides of the used gauze, the fcc lattice parameter (
a) and the size of coherent scattering region (
D) had close values equal to 3.903, 3.900 and 3.902 Å and 51, 74 and 39 nm, respectively [
21]. The obtained
a and
D values testified to the absence of both the dissolution of the detected C, O and N atoms in the alloy lattice with the formation of interstitial solutions and the significant merging of subgrains during the catalytic etching initiated by NH
3 oxidation. The highly exothermic oxidation of NH
3 led to the formation of “hotspot” etching sites on etch pits, which generated temperature gradients both on the surface [
6] and in the layer of agglomerates [
20]. The appearance of such gradients may have resulted in the mass transfer of metals from “hot” to “cold” regions of the catalyst during the surface diffusion of metal atoms or evaporation and condensation of volatile oxides such as PtO
2 and others, leading to deep etching of the surface with the formation of a rough layer of “cauliflowers”.
The surface and subsurface composition of the etched layer on the Pt–Pd–Rh–Ru gauze after its operation in NH
3 oxidation was investigated using XPS and energy-dispersive X-ray spectroscopy (EDS). SEM, EDS and XPS, which strongly differ in the effective depth of analysis [
23,
24], were applied to analyze the chemical composition of a ca. 5 nm-thick surface layer and the subsurface region to a depth up to ca. 500 nm on Pt–Pd–Rh–Ru gauzes used in the oxidation of NH
3 (see below Materials and Methods).
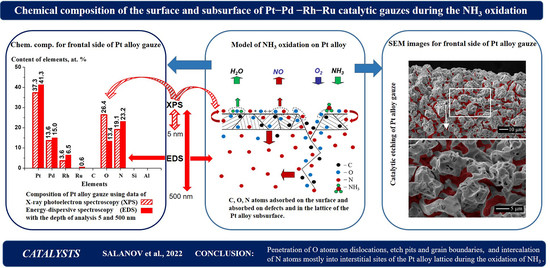
2.3. Spectral Characteristics, Chemical State and Concentration of Elements on the Surface of Fresh Pt–Pd–Rh–Ru Gauze and Both Sides of the Gauze after NH3 Oxidation according to XPS Data
XPS spectra were recorded for the fresh Pt–Pd–Rh–Ru gauze and for both sides of such gauze after NH
3 oxidation at 1133 K. The XPS spectrum for the fresh gauze is displayed in
Figure 3a; close spectra with additional N and Si peaks were obtained for the spent gauzes. These spectra were used to determine the elemental composition, chemical state of atoms and their concentration in a ca. 5 nm-thick surface layer. On the frontal side of the spent gauze, XPS spectra for Pt, Pd, Rh, C, O, N and Si, corresponding to Pt4
f, Pd3
d, Rh3
d, C1
s, O1
s, N1
s and Si2
p electronic levels, respectively, were recorded. XPS peaks of Ru were not detected, probably due to its low concentration in subsurface layers of the alloy, below the detection limit of the XPS method. The recorded spectra were also used to estimate the binding energy (
Eb) of Pt4
f7/2 (71.1 eV), Pd3
d5/2 (335.5 eV), C1
s (285.1 eV) and Si2
p (102.6 eV) levels. Two peaks were observed in Rh3
d5/2, C1
s, O1
s and N1
s spectra, so these spectra were deconvoluted by the Gauss method to distinguish two peaks that are necessary for refining the
Eb values and estimating the area of the peaks.
Figure 4 displays XPS spectra of Rh3
d5/2 (a,d,g), O1
s (b,e,h) and N1
s (c,f,i) with the peaks separated by deconvolution of these spectra for the fresh gauze (a,b,c) and also for the back (d,e,f) and frontal (g,h,i) sides of the gauze used in the oxidation of NH
3. C1
s spectra of the tested gauzes were quite similar to each other, so they are not shown in
Figure 4.
For the frontal side of the used gauze, deconvolution of the Rh3
d5/2 spectrum gave two peaks with close intensities and
Eb values equal to 307.2 and 308.4 eV (
Figure 4g). Deconvolution of O1
s and N1
s spectra gave two peaks with different intensity and
Eb values of 530.0 and 532.4 eV and 398.5 and 400.0 eV, as seen in
Figure 4h,i, respectively. Taking into account data reported in [
25], XPS peaks obtained in this study were used to reveal the chemical state of atoms of the detected elements. For Pt4
f7/2, Pd3
d5/2 and Rh3
d5/2 peaks, the obtained
Eb values equal to 71.1, 335.5 and 307.2 eV, respectively, testified to the metallic state of these elements (Pt
0, Pd
0 and Rh
0). According to [
25], for neat Pt, Pd and Rh, the
Eb values of these peaks are close to 71.2, 335.1 and 307.2 eV, respectively, while in various platinum, palladium and rhodium compounds,
Eb is higher than 72.0, 336.0 and 308.0 eV, respectively. The obtained Rh3
d5/2 peak with
Eb = 308.4 eV (
Figure 4g) indicated the Rh
3+ ionic state of rhodium, probably as a component of Rh
2O
3 oxide. The intense C1
s peak with
Eb = 285.1 eV testified to the graphitic state of carbon because the
Eb value for neat carbon falls in the range of 284.0–285.0 eV, while in metal carbides and carbon compounds with other atoms, the value for carbon is
Eb ≤ 283.0 and >285.0 eV, respectively [
25]. It should be noted that a low-intensity XPS peak was observed in the recorded C1
s spectrum at high
Eb values (>287.0 eV). Deconvolution of the C1
s spectrum gave an intense peak with
Eb = 285.1 eV and a low-intensity peak with
Eb = 287.3 eV, which was located in the energy range of 284.0–291.0 eV. In [
13] it is noted that broad XPS peaks of C1
s may correspond to carbon compounds with different atoms. The XPS peak with
Eb = 287.3 eV may include the peaks corresponding to (C–O) and (C–N), for which the
Eb values are in the ranges of 286.0–291.5 and 285.2–288.2 eV, respectively [
25]. Note that the C1
s peak with
Eb = 287.3 eV could not be associated with the adsorbed carbonate groups, since for them, the
Eb value for the C1
s peak was higher and fell in the range of 289.0–291.5 eV. For the Si2
p peak, the obtained
Eb = 102.6 eV was close to the
Eb value corresponding to that in SiO
2 (103.3 eV). For the O1
s peak, the acquired
Eb = 530.0 eV (
Figure 4h) indicated the O
2− ionic state of oxygen in Rh
2O
3 oxide. The O1
s peak with
Eb = 532.4 eV (
Figure 4h) could be assigned to oxygen in the adsorbed hydroxyl groups (OH
ad) and carbon oxide molecules (CO
ad) because their
Eb value was between 531.0 and 532.0 eV; in addition, this peak may correspond to oxygen in SiO
2 particles, where
Eb for the O1
s peak is between 532.5 and 533.3 eV [
25]. Note also that the O1
s peak with
Eb = 532.4 eV could not be attributed to carbonate groups because their
Eb for the O1
s peak had a decreased value and fell in the range of 530.5–531.5 eV. The obtained N1
s peak with a binding energy 398.5 eV (
Figure 4i) could be assigned both to the adsorbed nitrogen atoms (N
ad) and to nitrogen in metal nitrides in the alloy because their
Eb for the N1
s peak ranged from 396.2 to 398.2 eV. The N1
s peak with
Eb = 400.0 eV (
Figure 4i) testified to nitrogen compounds with different atoms, particularly the (C–N)
ad groups and (NH
3)
ad molecules, for which
Eb values for the N1
s peak were in the ranges of 397.8–400.2 and 398.8–399.7 eV, respectively [
25]. The formation of such compounds is quite probable during the oxidation of NH
3.
On the fresh Pt–Pd–Rh–Ru gauze and on the back side of the spent gauze, XPS spectra recorded for Pt, Pd, Rh, C and O had peaks corresponding to Pt4
f, Pd3
d, Rh3
d, C1
s and O1
s electronic levels, respectively; in addition, for the back side, XPS spectra recorded for N and Si had peaks corresponding to N1
s and Si2
p levels, respectively. For these gauzes, as for the frontal side of the used gauze, spectral characteristics of the detected elements were revealed. XPS spectra of Rh3
d5/2, O1
s, C1
s and N1
s also contained two peaks; so these spectra were deconvoluted into two individual peaks, which were necessary for refining the
Eb values and estimating the areas of these peaks (
Figure 4a–f). C1
s spectra of the fresh gauze and back side of the spent one contained not only the intense peaks with
Eb~285.0 eV, but also the low-intensity XPS peaks at high
Eb values (>287.0 eV). Deconvolution of C1
s spectra gave the intense peaks with
Eb = 285.4 and 285.1 eV and the low-intensity peaks with
Eb = 286.1 and 287.6 eV for the fresh gauze and back side, respectively. Taking into account data for the frontal side of the used gauze, the obtained intense peaks at
Eb~285.0 eV were attributed to graphitic carbon, while the low-intensity peaks at
Eb~286.0–288.0 eV may have been associated with (C–O) and (C–N) groups.
For the fresh Pt–Pd–Rh–Ru gauze and for both sides of the spent gauze, relative concentrations (at.%) of Pt, Pd, Rh, C, O, N and Si in a ca. 5 nm-thick surface layer of the gauzes were calculated from the measured areas of peaks in XPS spectra of Pt4
f, Pd3
d, Rh3
d5/2, C1
s, O1
s, N1
s and Si2
p, respectively, using atomic sensitivity factors of these elements [
25]. The 4
f7/2, 4
f5/2 and 3
d5/2, 3
d3/2 peaks were used for Pt and Pd, respectively, while for rhodium, only the 3
d5/2 peak was used because the Rh3
d3/2 peak overlapped the intense Pt4
d5/2 peak. In addition, for Rh, O, N and C we used the peaks obtained by deconvolution of Rh3
d5/2, O1
s, N1
s and C1
s spectra, respectively, as seen in
Figure 4.
Table 1 lists the
Eb values obtained for individual electronic levels and the concentrations for the detected elements (Pt, Pd, Rh, C, O, N and Si), particularly those calculated from the peaks obtained by deconvolution of Rh3
d5/2, O1
s, N1
s and C1
s spectra for the fresh Pt–Pd–Rh–Ru gauze (a,b) and also for the back (c,d) and frontal (e,f) sides of such gauze used in the oxidation of NH
3.
Data in
Table 1 demonstrated an essential difference in the concentrations of detected elements. For all the gauzes, carbon and oxygen were detected in high concentrations equal to 57.9, 48.4 and 56.3 at.% for C and 29.8, 35.3 and 31.1 at.% for O, whereas the other elements had much lower concentrations ranging from 1.0 to 5.0 at.%. Thus, the XPS study detected Pt, Pd, Rh, C and O in a ca. 5 nm-thick surface layer on the new Pt–Pd–Rh–Ru gauze, whereas Pt, Pd, Rh, C, O, N and Si were observed on both sides (back and frontal) of the spent gauze. Two XPS peaks were revealed for Rh, C, O and N, which indicated different states of atoms for these elements. This suggests that on all the gauzes, the surface layer with a thickness of ca. 5 nm contained the metallic alloy of Pt
0, Pd
0 and Rh
0 atoms, Rh
2O
3 oxide, graphitic carbon and the adsorbed layer of OH
ad and CO
ad. The surface layer of the gauzes had high concentrations of carbon and oxygen (48.4–57.9 at.% for C and 29.8–35.3 at.% for O) and low concentrations of the other elements (≤5 at.%). The data obtained were used to elucidate the composition and structure of the surface films on the gauzes under consideration.
2.4. Composition and Structure of the Surface Films on the Fresh Pt–Pd–Rh–Ru Gauze and Both Sides of the Gauze Used in NH3 Oxidation according to XPS Data
Figure 5 shows the concentrations of elements measured by XPS in a ca. 5 nm-thick surface layer on the new Pt–Pd–Rh–Ru gauze (a–d) and also on the back (e–h) and frontal (i–l) sides of such gauze used in the oxidation of NH
3 at 1133 K.
Figure 5a,e,i display the concentrations listed in
Table 1 of all the detected elements, namely, Pt, Pd, Rh, C and O for the new gauze (
Figure 5a) and Pt, Pd, Rh, C, O, N and Si for both sides of the used gauze (
Figure 5e,i), respectively. For all the gauzes, XPS peaks corresponding to Ru were not detected, probably due to the low Ru concentration in the surface layers of the tested gauzes. For both sides of the spent gauze, Si was detected in the concentrations of 4.6 and 4.1 at.%, and N–4.1 and 2.1 at.%, respectively (
Figure 5e,i). The presence of Si may indicate that SiO
2 particles were transferred to gauzes from the quartz reactor, while the presence of N may testify to the accumulation of nitrogen atoms in the catalyst surface layers during the oxidation of NH
3.
On the new gauze and on both sides of the gauze after its operation in NH
3 oxidation, the following contents of elements were obtained: Pt—4.6, 4.9 and 4.1 at.%; Pd—2.4, 1.9 and 1.5 at.%; and Rh—5.3, 0.8 and 0.8 at.%, respectively (
Table 1b,d,f and
Figure 5a,e,i). According to XPS spectra, all the detected Pt and Pd atoms and a part of the Rh atoms in the concentrations of 0.1, 0.4 and 0.4 at.% entered the composition of metallic alloy on these gauzes (
Table 1b,d,f). The remaining part of the Rh atoms in the ionic form Rh
3+ could enter the composition of Rh
2O
3 oxide. The new gauze had an increased content of rhodium (5.3 at.%) in comparison with both sides of the spent gauze (0.8 and 0.8 at.%, respectively), as seen in
Figure 5a,e,i. The surface enrichment of the new gauze with rhodium may be related to the pretreatment of gauzes before mounting in the reactor. The obtained Rh3
d5/2 peaks with
Eb of 308.4–308.6 eV (
Figure 4a,d,g) testified to Rh
3+ in the concentrations of 5.2, 0.4 and 0.4 at.% (
Table 1b,d,f). The O1
s peaks with
Eb of 529.9–530.2 eV (
Figure 4b,e,h) and concentrations of 5.3, 6.2 and 3.5 at.% (
Table 1b,d,f), taking into account the data for Rh
3+, may have included O
2− ions in the concentrations of 5.3, 0.6 and 0.6 at.% in Rh
2O
3. The O1
s and Rh3
d5/2 peaks assigned to O
2− and Rh
3+ indicated the presence of Rh
2O
3 oxide film on the alloy surface of all gauzes, which is consistent with the data of Ref. [
10]. In this work, the Rh
2O
3 surface film with a thickness below 2 nm was detected on a Pt–10 wt.% Rh gauze after NH
3 oxidation. Such an oxide film can form upon cooling the gauzes after termination of the catalytic process because Rh
2O
3 oxide becomes unstable under the conditions of NH
3 oxidation [
10].
A close carbon content equal to 57.9, 48.4 and 56.3 at.%, respectively, was obtained on the tested gauzes (
Figure 5a,e,i). The solubility of carbon in platinum with the formation of solid interstitial solutions is extremely low [
26]. In this context, close
Eb values (285.1–285.4 eV) for C1
s peaks in the concentrations of 49.1, 37.9 and 45.9 at.% testified to the surface graphitic state of carbon on all the gauzes (
Table 1b,d,f). Carbon detected in the surface layer of the new Pt–Pd–Rh–Ru gauze and Pt(poly), which had
Eb = 285.4 and 284.5 eV for C1
s, was attributed to surface graphitic films with a thickness of ca. 10 nm [
18] and 1–2 nm [
27], respectively. XPS data obtained in our study showed the presence of graphitic carbon film (C
gr) with a close thickness of 1–10 nm on all the gauzes under consideration. In addition, it should be noted that the concentrations of carbon indicated in
Figure 5a,e,i included not only C
gr, but also other states of carbon: C
ab, (C–O)
ab and (C–N)
ab (see below).
The fresh and spent gauzes (back and frontal sides) were shown to have a close content of oxygen: 29.8, 35.3 and 31.1 at.%, respectively (
Figure 5a,e,i). The O1
s peak with
Eb = 532.2, 532.5 and 532.4 eV (
Figure 4b,e,h) corresponded to oxygen in the concentrations of 24.5, 29.1 and 27.6 at.%, respectively (
Table 1b,d,f), in the adsorbed surface layer of OH
ad and CO
ad, particularly in the composition of SiO
2. It should be noted that the oxygen concentrations shown in
Figure 5a,e,i included, along with the surface layer (OH
ad, CO
ad and SiO
2), oxygen of Rh
2O
3 oxide and oxygen atoms intercalated on defects (O
ab) (see below). It should be noted also that the obtained concentrations of C and O on all the gauzes and N concentrations on the spent gauzes (
Figure 5a,e,i) were close to the concentrations of these elements after NH
3 oxidation in an industrial reactor for several weeks on a pack of 36 Pt–10 wt.% Rh gauzes [
9]. According to XPS, on gauzes 2, 16, 31 and 36 of this pack, the concentrations of C were equal to 49.3, 49.1, 47.1 and 52.5 at.%, for O—25.6, 35.0, 30.5 and 27.7 at.% and for N—5.4, 2.0, 4.2 and 2.8 at.%, respectively.
Thus, according to XPS, the oxide, graphitic carbon and adsorption surface films were detected on the new Pt–Pd–Rh–Ru gauze and on both sides of the spent gauze. On the spent gauzes, the oxide film (Rh2O3) with a thickness ≤2 nm may have formed on the metallic alloy surface upon cooling the reactor. The graphitic carbon film (Cgr) with a thickness from 1 to 10 nm may have formed on the oxide film surface in air. The adsorption surface layer (OHad and COad) formed on the Cgr surface, also in air. Data on the composition and structure of the observed surface films on the platinum alloy gauzes studied in this work were used to reveal the composition of the catalyst surface under such films.
2.5. Chemical Composition of the Catalyst Surface under the Adsorption, Carbon and Oxide Films for Fresh and Spent Pt–Pd–Rh–Ru Gauzes according to XPS Data
Figure 6 displays a scheme of the surface and subsurface layers of the Pt–Pd–Rh–Ru alloy used in the oxidation of NH
3. The surface adsorption layer (OH
ad, CO
ad) as well as the graphitic carbon and oxide films (C
gr, Rh
2O
3) are indicated on the scheme. In addition, the scheme also shows the C
ab, O
ab and N
ab atoms absorbed on grain boundaries and other defects in the subsurface layer of metallic alloy. Dotted ellipses indicate relative depths of analysis by XPS (ca. 5 nm) and EDS (ca. 500 nm). Moreover, these methods were shown to exert different effects on the adsorption layer (OH
ad, CO
ad). XPS produced virtually no effect, whereas EDS led to complete destruction of the layer. The surface films (OH
ad, CO
ad, C
gr, Rh
2O
3 and SiO
2) that were observed on the Pt–Pd–Rh–Ru gauzes examined in this study were formed mostly after the oxidation of NH
3 upon cooling the reactor and in air, when the sample was transferred from the reactor to the microscope and spectrometer.
With such films, quantitative analysis of the composition may give a significant deviation of the obtained surface concentrations of elements from their real content. It becomes difficult to estimate the concentrations of elements on the catalyst surface. This suggests that the measured composition of Pt–Pd–Rh–Ru gauzes with the surface films emerging after NH
3 oxidation will differ essentially from the surface composition of the catalyst that forms during the oxidation of NH
3. In this context, it seems interesting to estimate the surface composition of gauzes under the revealed surface films (OH
ad, CO
ad, C
gr, Rh
2O
3 and SiO
2;
Figure 6). Under these films, the concentrations of elements (Pt
0, Pd
0, Rh
0, C
ab, O
ab, N
ab) in the surface layer of gauzes were calculated from the obtained XPS data, taking into account the XPS peaks resolved by deconvolution of spectra and the stoichiometry of Rh
2O
3 oxide (
Figure 4 and
Table 1).
Table 1 lists XPS data on the concentrations of elements in a ca. 5 nm-thick surface layer on the new Pt–Pd–Rh–Ru gauze and both sides (back and frontal) of the spent gauze. All the detected Pt (4.6, 4.9, 4.1 at.%) and Pd (2.4, 1.9, 1.5 at.%) atoms as well as a part of Rh (0.1, 0.4 and 0.4 at.%) atoms entered the composition of the metallic alloy (
Table 1b,d,f). These data testified to the presence of metallic alloy (Pt
0, Pd
0 and Rh
0) on the surface of gauzes under the surface films (OH
ad, CO
ad, C
gr, Rh
2O
3 and SiO
2).
For all the tested gauzes, C1
s spectra, along with the intense peaks with
Eb = 285.1–285.4 eV corresponding to C
gr film, at high
Eb values (286.1–287.6 eV) contained low-intensity XPS peaks. Deconvolution of C1
s spectra gave the intense peaks with
Eb = 285.4, 285.1, 285.1 eV and the low-intensity peaks with
Eb = 286.1, 287.6, 287.3 eV for the new gauze and both sides of the used gauze, respectively. For all the gauzes, C1
s peaks obtained with
Eb ≥ 286.1 eV for carbon atoms in the concentrations of 8.8, 10.5 and 10.4 at.% (
Table 1b,d,f) could be attributed to the absorbed carbon atoms (C
ab) and C atoms in the composition of (C–O)
ab and (C–N)
ab on the surface and in subsurface layers of the alloy under the revealed surface films. C
ab atoms can reside on such defects as dislocations and grain boundaries (
Figure 6). Unfortunately, it was impossible to separate the peaks corresponding to C
ab, (C–O)
ab and (C–N)
ab; so the concentration of C
ab was estimated from C1
s peaks with
Eb = 286.1, 287.6 and 287.3 eV. As a result, C atoms in the concentrations of 8.8, 10.5 and 10.4 at.% for the fresh and spent gauzes, respectively (
Table 1b,d,f), were attributed to the absorbed carbon atoms (C
ab) under the surface films in metallic alloy. It should be noted that according to AES data for Pt–Pd–Rh gauzes, carbon was detected on the surface and in subsurface layers at a depth of 4 and 12 nm in the concentrations of 45.8, 7.9 and 4.1 at.% on the new gauze and 58.2, 29.1 and 12.4 at.% on the spent one, respectively [
11]. The data obtained in this work at a depth of 4 and 12 nm satisfactorily agreed with our estimates of the C
ab concentrations in metallic alloy of the Pt–Pd–Rh–Ru gauze under the surface films.
For all the gauzes under consideration, O1
s spectra, along with intense peaks with
Eb = 532.2–532.5 eV corresponding to OH
ad, CO
ad and SiO
2, at lower
Eb values (529.9–530.2 eV) contained XPS peaks (
Figure 4b,e,h) that were assigned to the O
2− state in the surface film of Rh
2O
3 oxide. In addition, this oxygen could be assigned to the atoms chemisorbed on the surface (O
ad) or penetrated on dislocations and grain boundaries (O
ab) in the metallic alloy under the surface films, because in metal oxides and in the chemisorbed state,
Eb for oxygen is ≤531.0 eV [
25]. Hence, oxygen in the concentrations of 5.3, 6.2 and 3.5 at.% (
Table 1b,d,f) may have included O
2− in the Rh
2O
3 oxide film and O
ab atoms on defects in subsurface layers of the catalyst. Taking into account oxygen in Rh
2O
3 oxide for the back and frontal sides of the used gauze (0.6 and 0.6 at.%), their O
ab concentrations became equal to 5.6 and 2.9 at.%. For the new gauze, thick surface oxide and carbon films shielded the metallic alloy, which made it difficult to measure the O
ab concentration in its surface layer. In this context, the O
ab concentration in the surface metallic alloy of the new gauze was taken to be equal to the average value obtained for both sides of the spent gauze (4.3 at.%). Thus, the O atoms in the concentrations of 4.3, 5.6 and 2.9 at.% were attributed to the absorbed oxygen atoms on defects (O
ab) in the metallic alloy under the surface films. In [
27], the accumulation of 5–10 at.% oxygen atoms on grain boundaries and other defects of Pt(poly) were observed at
T = 600–1400 K and
Po
2~2 × 10
4 Pa; dissolution of O atoms in the platinum lattice was not observed. Thus, it can be accepted that O atoms do not dissolve in the lattice of the alloy with the predominant content of Pt, which was examined in our study; these atoms are accumulated mostly on such defects as dislocations and grain boundaries. Moreover, it should be noted that the obtained O
ab concentrations were consistent with the data acquired on Pt–Pd–Rh gauzes [
11]. According to AES data, the O concentrations on the surface and in subsurface layers of the gauzes at depths of 4 and 12 nm were equal to 17.2, 9.8 and 3.4 at.% for the new gauze, and 5.7, 8.0 and 6.1 at.% for the spent gauze, respectively.
On both sides of the gauze used in NH
3 oxidation, nitrogen detected in the concentrations of 4.1 and 2.1 at.% (
Figure 5e,i) was characterized by two XPS peaks with
Eb equal to 397.8–398.5 eV and 399.7–400.0 eV (
Figure 4f,i), respectively. On the new gauze, nitrogen was detected at the noise level (
Figure 4c). The obtained N1
s peaks could be attributed to nitrogen atoms, particularly in nitrides of the employed metals in the concentrations of 0.2 and 0.8 at.% and in the composition of (C–N), (NH
X) and (N–O) groups—3.9 and 1.3 at.%, respectively (
Table 1d,f), since the obtained
Eb values for the peaks correspond to such compounds [
13,
25]. Taking into account close
Eb values for these states, nitrogen in the concentrations of 4.1 and 2.1 at.% was assigned to the absorbed nitrogen atoms (N
ab) in metallic alloy under the surface films on the back and frontal sides of the spent gauze, respectively. During the oxidation of NH
3, N
ad atoms could penetrate on the defects, gradually accumulate in the surface layers of metallic alloy and form bonds both with metal atoms and with C
ab and O
ab. It should be noted that the obtained N concentrations on the Pt–Pd–Rh–Ru gauzes were close to the values for gauzes 2, 16, 31 and 36 in a pack of 36 Pt–10 wt.% Rh gauzes after NH
3 oxidation in an industrial reactor [
9]. According to XPS data, on these gauzes the concentrations of N were equal to 5.4, 2.0, 4.2 and 2.8 at.%, respectively.
The obtained data on the concentrations of metal atoms in the alloy (Pt
0, Pd
0, Rh
0) and absorbed atoms (C
ab, O
ab, N
ab) in such alloy under the surface films were used to calculate new values of the relative concentrations for Pt
0, Pd
0, Rh
0, C
ab, O
ab and N
ab elements on the gauze surface. After excluding the surface films (OH
ad, CO
ad, C
gr, Rh
2O
3 and SiO
2) (
Figure 6) from the calculation, the following concentrations of elements (at.%) in the metallic alloy were obtained: for the new gauze—Pt
0 (4.6), Pd
0 (2.4), Rh
0 (0.1), C
ab (8.8), O
ab (4.3), N
ab (0.0); for the back and frontal sides of the spent gauze—4.9, 1.9, 0.4, 10.5, 5.6, 4.1 and 4.1, 1.5, 0.4, 10.4, 2.9, 2.1, respectively. New values of the relative concentrations of elements under the surface films were calculated from the indicated data (
Figure 5b,f,j). For both sides of the used gauze, the concentrations of C
ab, O
ab and N
ab were equal to 38.3, 20.4 and 15.0, and 48.5, 13.6 and 9.8 at.%, respectively (
Figure 5f,j). According to AES data, on the fresh Pt–Pd–Rh gauze and on the gauze after the operation in NH
3 oxidation, the surface concentrations of C were equal to 45.8 and 58.2 at.%, respectively [
11]; these values agreed with the data obtained in our study (
Figure 5a,e,i). For the spent gauze, the concentrations of C and O in the subsurface layer at a depth of 4 nm was 29.1 and 8.0 at.%, respectively [
11]. These values were somewhat lower than the concentrations obtained in our study under the surface films on the spent gauze (
Figure 5f,j). Note that in [
11] the effect of the carbon film on the obtained concentrations of elements was not considered; in addition, nitrogen was not detected in it. It seems interesting also that EDS revealed a considerable deviation in the calculated concentrations of C that were obtained on different surface regions of these gauzes (see below). This may have led to a significant deviation of the obtained concentrations of other elements from their real content in the surface layer of the catalyst. Thus, to estimate the concentrations of elements under the surface films, carbon was completely excluded from the list of detected elements. New values of the relative concentrations of elements were derived from the data of
Figure 5b,f,j after excluding C
ab from the calculation (
Figure 5c,g,k). For the back and frontal sides of the spent gauze, the concentrations of O
ab and N
ab in the metallic alloy without C
ab atoms under the surface films were equal to 33.1 and 24.3, and 26.4 and 19.1 at.%, respectively (
Figure 5g,k).
Using the data of
Figure 5c,g,k and excluding O
ab and N
ab from the calculation, new values of the relative concentrations of metals under surface films were obtained (
Figure 5d,h,l). For the new gauze, we observed a minor decrease in the content of Pt
0 (64.8 at.%), a noticeable excess of Pd
0 (33.8 at.%), and a considerable deficit of Rh
0 (1.4 at.%) in comparison with both sides of the gauze used in NH
3 oxidation, for which Pt had concentrations of 68.1 and 68.3 at.%, Pd—26.4 and 25.0 at.% and Rh—5.5 and 6.7 at.%, respectively (
Figure 5d,h,l). Presumably, during the pretreatment of new gauzes, Rh
0 atoms migrate from subsurface layers to the surface, while Pd
0 atoms are segregated from deeper layers of the alloy to subsurface layers. The formation of a thick surface Rh
2O
3 oxide film is accompanied by depletion with rhodium and enrichment with palladium of the subsurface layer of metallic alloy under the oxide film. For both sides of the used gauze, the surface content of Pt
0, Pd
0 and Rh
0 (
Figure 5h,l) virtually coincided with each other and with the data provided by the manufacturer: Pt (69.8), Pd (23.7) and Rh (5.7 at.%).
Thus, the surface composition of Pt–Pd–Rh–Ru gauzes under the surface films (OHad, COad, Cgr, Rh2O3 and SiO2) was estimated from XPS data with a ca. 5 nm depth of analysis. For the spent gauze, a metallic alloy with the composition close to that claimed by the gauze manufacturer was found under such films. The metallic alloy contained Cab, Oab and Nab atoms absorbed on dislocations, grain boundaries and others; their concentrations were 38.3, 20.4 and 15.0 at.% on the back side and 48.5, 13.6 and 9.8 at.% on the frontal side of the spent gauze, respectively. After excluding Cab from the calculation, the concentrations of Oab and Nab in the alloy increased and became equal to 33.1 and 24.3 at.%, and 26.4 and 19.1 at.% on both sides, respectively. These data testified to higher concentrations of Oab atoms as compared to Nab in surface layers of the metallic alloy under the surface films. Data on the catalyst surface composition under the surface films obtained by XPS with the analysis depth of ca. 5 nm were used for comparison with the composition of the catalyst subsurface layers estimated by EDS with the analysis depth of ca. 500 nm.
2.6. Chemical Composition of Subsurface Layers of the Fresh and Spent Pt–Pd–Rh–Ru Gauzes according to EDS Data
The EDS method was used to determine the elemental composition of subsurface layers of the wire (to a depth up to ca. 500 nm) for the new Pt–Pd–Rh–Ru gauze and both (back and frontal) sides of the gauze after its operation in the oxidation of NH3 at 1133 K. On all the gauzes, the elemental composition of the subsurface layer was estimated using the wire fragments with a length of ca. 800 µm in three rectangular regions with sizes of 60 × 200 µm. On all the gauzes, Pt, Pd, Rh, Ru, C, O and Al elements were detected; in addition, N was detected on both sides of the spent gauze. On all the gauzes, the concentrations of Al and C widely varied from one region to another, for example, on the frontal side, from 0.5 to 1.3 at.% for Al (the average value of ca. 1.0 at.%), and from 24.4 to 61.9 at.% for C (ca. 45.1 at.%), respectively. Concentrations of other elements in these regions differed from each other to a smaller extent. The average Al and C concentrations on the new gauze and both sides of the used gauze were equal to 0.2, 0.2 and 1.0 at.% for Al and 44.4, 56.7 and 45.1 at.% for C, respectively.
Table 2 lists the average concentrations of elements calculated from EDS measurements on the frontal side (a,b) and according to the data provided by the manufacturer (c).
Table 2 indicates the concentrations of all the detected elements (a) and the relative concentrations of metals calculated after exclusion of C, O, N and Al from the list of detected elements (b).
The obtained average concentrations of all the detected elements showed increased concentrations for C (45.1 at.%) and Pt (22.3 at.%) and much lower values for other elements (<12.5 at.%) (
Table 2a). After excluding C, O, N and Al from the list of elements, the obtained content of metals (
Table 2b) generally approached the bulk composition of the alloy claimed by the manufacturer (
Table 2c). An increased concentration of Rh in the tested gauze (10.6 at.%) as compared to the manufacturer data (5.7 at.%) may have resulted from rhodium accumulation in the subsurface layers of the wire during the oxidation of NH
3. In [
8,
9,
10], on Pt–Rh gauzes used in the industrial oxidation of NH
3, the surface concentration of Rh measured by XPS was higher than its bulk concentration.
According to EDS measurements of the concentrations of elements,
Figure 7 shows the content of elements on the fresh Pt–Pd–Rh–Ru gauze (a,b,c) and also on the back (d,e,f) and frontal (g,h,i) sides of the gauze after NH
3 oxidation.
Figure 7a,d,g indicate the concentrations of all the detected elements on these gauzes.
Figure 7a,d,g show a low content of Ru (0.4, 0.2 and 0.3 at.%) and Al (0.2, 0.2 and 1.0 at.%) for all the gauzes. Ru had a low concentration in the alloy (0.8 at.%,
Table 2c) and may have been distributed non-uniformly over the wire volume; small amounts of Al may have appeared in the wire during its production. The detected metals (Pt, Pd, Rh) generally had close concentrations in the tested gauzes: Pt—25.8, 21.9, 22.3 at.%; Pd—12.7, 7.8, 8.1 at.%; and Rh—3.3, 1.7, 3.5 at.%, respectively (
Figure 7a,d,g). Only an increased content of Pd was observed on the new gauze (12.7 at.%) in comparison with both sides of the spent gauze (7.8 and 8.1 at.%). For oxygen detected on all the gauzes, the concentration was slightly higher on the fresh gauze (13.2 at.%) than on both sides (4.9 and 7.2 at.%), respectively (
Figure 7a,d,g). Taking into account XPS data (see above), a part of the detected oxygen could enter the composition of Rh
2O
3 oxide film on the surface of metallic alloy. According to XPS data, the oxide film had a much greater thickness on the new gauze as compared to both sides of the used gauze. In this context, the increased concentration of oxygen revealed by EDS on the new gauze may have been associated with the oxide film on the alloy surface. In addition, according to XPS data, a part of the oxygen atoms resided on defects in the subsurface layer of the alloy (O
ab). A quite close content of carbon (44.4, 56.7, 45.1 at.%) was observed for all the gauzes (
Figure 7a,d,g). These data indicated the presence of the graphitic carbon film (C
gr) revealed by XPS, which was formed on the surface of oxide film when samples were transferred in air from the reactor to the microscope (
Figure 6). The adsorbed layer of OH
ad and CO
ad on the carbon film surface, which was detected by XPS, decomposed to a great extent under the action of the microscope probe electrons, so oxygen in the adsorbed molecules was not detected by EDS. Nitrogen was detected on both sides of the gauze after NH
3 oxidation in the concentrations of 6.6 and 12.5 at.% (
Figure 7d,g), whereas on the fresh gauze, N was not detected (
Figure 7a). This testified to penetration of N atoms in the subsurface layer of the wire during NH
3 oxidation. It should be noted also that the elements detected by EDS included all states of these elements. The C atoms detected by EDS entered the composition of graphitic film (C
gr) and resided on defects in subsurface layers of carbon (C
ab), and the detected O atoms were present in Rh
2O
3 oxide and in O
ab atoms absorbed on defects. In this context, EDS data could not be used to estimate the concentrations of C
ab and O
ab in subsurface layers of the alloy separately from C
gr and Rh
2O
3.
The concentrations of Pt, Pd, Rh, Ru and O
ab, as well as N
ab, in subsurface layers of the alloy were also calculated from EDS data taking into account the surface films (OH
ad, CO
ad, C
gr, Rh
2O
3) detected earlier by XPS (
Figure 6). The adsorbed layer (OH
ad and CO
ad) decomposed under the action of the microscope probe electrons, so its effect on the concentrations of elements on the gauze surface was negligible. The Rh
2O
3 surface oxide film had a small thickness, especially for the spent gauze (<2 nm), because it had low concentrations of oxygen (4.9 and 7.2 at.% (
Figure 7d,g)). The depth of EDS analysis (ca. 500 nm) suggested that such a film could exert only a slight effect on the concentrations of elements measured by EDS. In this context, the oxide film was not taken into account when estimating the concentrations of elements in subsurface region of the catalyst. Thus, the obtained concentrations of oxygen (13.2, 4.9 and 7.2 at.% (
Figure 7a,d,g)) were attributed to the O atoms absorbed on defects in subsurface layers of the gauzes (O
ab). Graphitic carbon film (C
gr) on the alloy surface strongly affected the results of quantitative analysis of elements because high concentrations of carbon were observed on all the gauzes (44.4, 56.7, 45.1 at.%) (
Figure 7a,d,g). Moreover, carbon could additionally accumulate on the surface of this film upon recording the EDS spectra. Note also that quantitative determination of the carbon content with the use of EDS spectra for the tested alloy was hindered by the overlapping of the C
Kα
1,2 and
Mζ peaks for Pd, Rh and Ru used for quantitative analysis. The above listed factors produced considerable deviations in the C concentrations calculated using EDS data from the real content of carbon in the catalyst. This may have exerted a considerable effect on the measured concentrations of other elements. Thus, for quantitative analysis of elements, carbon as the component of C
gr and C
ab was excluded from the list of detected elements.
New values of the relative concentrations of Pt, Pd, Rh, Ru, O and N were calculated from the data of
Figure 7a,d,g after excluding C and Al.
Figure 7b,e,h indicate the obtained concentrations of these elements. In the metallic alloy, O
ab and N
ab atoms absorbed in the subsurface layer had concentrations of 11.4 and 15.3 at.% on the back side and 13.4 and 23.2 at.% on the frontal side of the spent gauze, respectively (
Figure 7e,h). Note that the obtained concentration of N was somewhat higher than that for oxygen; this was most pronounced on the frontal side, for which the N/O ratio was equal to 1.73. This may testify that in comparison with oxygen, N atoms dissolve more intensely in the subsurface layer of metallic alloy during the oxidation of NH
3 on both sides of the gauze. Taking into account that EDS detects all of the oxygen, particularly that in the oxide film and O
ab, an excess of N
ab with respect to O
ab may be even greater. Thus, for the frontal side, the N/O ratio may reach ca. 2.0 and even higher values. Relative concentrations of Pt, Pd, Rh and Ru metals were obtained from the data of
Figure 7b,e,h after excluding O and N from the calculation (
Figure 7c,f,i). For the new gauze, an excess of Pd (30.0 at.%) over the gauze manufacturer data (23.7 at.%,
Table 2c) was found. For the back side, the obtained concentrations of metals were close to the contents of these elements claimed by the manufacturer: Pt (69.8), Pd (23.7), Rh (5.7) and Ru (0.8 at.%) (
Table 2c). For the frontal side, an increased content of Rh (10.3 at.%) in comparison with the manufacturer data (5.7 at.%) was obtained. The accumulation of Rh in subsurface layers of the alloy may have been related to the temperature elevation due to the more intense oxidation of NH
3 on the frontal side of the gauze as compared to its back side. On Pt–Rh gauzes used in the industrial oxidation of NH
3, the surface concentration of Rh was higher than its concentration in the bulk [
8,
9,
10].
Thus, EDS with the analysis depth of ca. 500 nm was used to obtain data on the composition of subsurface layers of Pt–Pd–Rh–Ru gauzes taking into account the surface films (Cgr and Rh2O3). After the exclusion of C and Al from the list of elements under consideration, the concentrations of Oab and Nab atoms absorbed in the subsurface region of the alloy became equal to 11.4 and 15.3 at.% on the back side and 13.4 and 23.2 at.% on the frontal side of the spent gauze, respectively. Close concentrations were obtained for Oab and Nab on the back side, while on the frontal side, the concentration of Nab exceeded nearly twofold that of Oab. This indicates a more intense dissolution of N atoms as compared to O in the subsurface region of metallic alloy on the frontal side, which had a higher temperature than the back side. The composition of metallic alloy for the back side coincided with the gauze manufacturer data, whereas for the frontal side, an excess of Rh was observed in comparison with the back side.