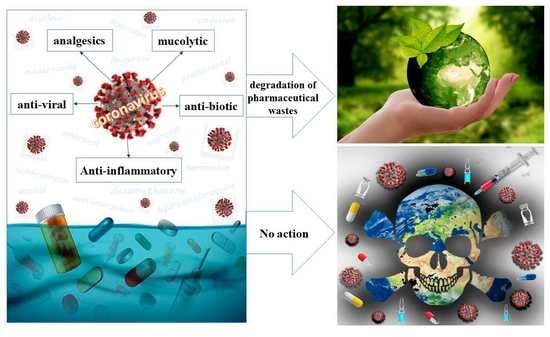
Nanomaterials for Photocatalytic Degradations of Analgesic, Mucolytic and Anti-Biotic/Viral/Inflammatory Drugs Widely Used in Controlling SARS-CoV-2
Abstract
:1. Introduction
2. Analgesics
2.1. Acetaminophen
One of the most frequently recommended pain relievers is non-steroidal anti-inflammatory drugs (NSAID) [64]. In soil, surface water, wastewater, groundwater, Antarctic ice, snow, sediments, and drinking water, NSAIDs have been detected in nanograms and micrograms. NSAIDs have long-term ecotoxic impacts on ecosystems, despite their low observable levels in the environment [65].
2.2. Aspirin
2.3. Ibuprofen
2.4. Naproxen
3. Mucolytics
Ambroxol
4. Antibiotics
4.1. Azithromycin
4.2. Quinolones
4.2.1. Hydroxychloroquine
4.2.2. Chloroquine Phosphate
5. Anti-Inflammatory Glucocorticoids
5.1. Dexamethasone
5.2. Cortisone Acetate
6. Antihistamines
Diphenhydramine
7. H2 Blockers
Famotidine
8. Anthelmintics
Praziquantel
9. Antivirals
9.1. Ivermectin
9.2. Acyclovir
9.3. Lopinavir/Ritonavir
9.4. Favipiravir
9.5. Nitazoxanide
9.6. Remdesivir
10. Outlook and Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Finnerup, N.B. Nonnarcotic Methods of Pain Management. N. Engl. J. Med. 2019, 18, 61. [Google Scholar] [CrossRef] [PubMed]
- Bakshani, C.R.; Morales-Garcia, A.L.; Althaus, M.; Wilcox, M.D.; Pearson, J.P.; Bythell, J.C.; Burgess, J.G. Evolutionary Conservation of the Antimicrobial Function of Mucus: A First Defence against Infection. NPJ Biofilms Microb. 2018, 4, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kapoor, G.; Saigal, S.; Elongavan, A. Action and Resistance Mechanisms of Antibiotics: A Guide for Clinicians. J. Anaesthesiol. Clin. Pharmacol. 2017, 33, 300–305. [Google Scholar] [CrossRef] [PubMed]
- C Reygaert, W. An Overview of the Antimicrobial Resistance Mechanisms of Bacteria. AIMS Microbiol. 2018, 4, 482–501. [Google Scholar] [CrossRef]
- Ahmed, M.H.; Hassan, A. Dexamethasone for the Treatment of Coronavirus Disease (COVID-19): A Review. SN Compr. Clin. Med. 2020, 2, 2637–2646. [Google Scholar] [CrossRef]
- Villoutreix, B.O.; Krishnamoorthy, R.; Tamouza, R.; Leboyer, M.; Beaune, P. Chemoinformatic Analysis of Psychotropic and Antihistaminic Drugs in the Light of Experimental Anti-Sars-Cov-2 Activities. Adv. Appl. Bioinform. Chem. 2021, 14, 71–85. [Google Scholar] [CrossRef]
- Reznikov, L.R.; Norris, M.H.; Vashisht, R.; Bluhm, A.P.; Li, D.; Liao, Y.S.J.; Brown, A.; Butte, A.J.; Ostrov, D.A. Identification of Antiviral Antihistamines for COVID-19 Repurposing. Biochem. Biophys. Res. Commun. 2021, 538, 173–179. [Google Scholar] [CrossRef]
- Spiro, H.M. H2-Blockers. J. Clin. Gastroenterol. 1983, 5, 143–148. [Google Scholar] [CrossRef]
- Kausar, S.; Said Khan, F.; Ishaq Mujeeb Ur Rehman, M.; Akram, M.; Riaz, M.; Rasool, G.; Hamid Khan, A.; Saleem, I.; Shamim, S.; Malik, A. A Review: Mechanism of Action of Antiviral Drugs. Int. J. Immunopathol. Pharmacol. 2021, 35, 20587384211002621. [Google Scholar] [CrossRef]
- Zhou, L.J.; Li, J.; Zhang, Y.; Kong, L.; Jin, M.; Yang, X.; Wu, Q.L. Trends in the Occurrence and Risk Assessment of Antibiotics in Shallow Lakes in the Lower-Middle Reaches of the Yangtze River Basin, China. Ecotoxicol. Environ. Saf. 2019, 183, 109511. [Google Scholar] [CrossRef]
- Chen, X.; Lei, L.; Liu, S.; Han, J.; Li, R.; Men, J.; Li, L.; Wei, L.; Sheng, Y.; Yang, L.; et al. Occurrence and Risk Assessment of Pharmaceuticals and Personal Care Products (PPCPs) against COVID-19 in Lakes and WWTP-River-Estuary System in Wuhan, China. Sci. Total Environ. 2021, 792, 148352. [Google Scholar] [CrossRef] [PubMed]
- Morales-Paredes, C.A.; Rodríguez-Díaz, J.M.; Boluda-Botella, N. Pharmaceutical Compounds Used in the COVID-19 Pandemic: A Review of Their Presence in Water and Treatment Techniques for Their Elimination. Sci. Total Environ. 2022, 2021, 152691. [Google Scholar] [CrossRef] [PubMed]
- Boxall, A.B.A. The Environmental Side Effects of Medication. EMBO Rep. 2004, 5, 1110–1116. [Google Scholar] [CrossRef] [Green Version]
- Melander, R.J.; Melander, C. The Challenge of Overcoming Antibiotic Resistance: An Adjuvant Approach? ACS Infect. Dis. 2017, 3, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, K.; Li, C.; Dhangar, K.; Kumar, M. Predicted Occurrence, Ecotoxicological Risk and Environmentally Acquired Resistance of Antiviral Drugs Associated with COVID-19 in Environmental Waters. Sci. Total Environ. 2021, 776, 145740. [Google Scholar] [CrossRef]
- Holmes, E.C.; Hurt, A.C.; Dobbie, Z.; Clinch, B.; Oxford, J.S.; Piedra, P.A. Understanding the Impact of Resistance to Influenza Antivirals. Clin. Microbiol. Rev. 2021, 34, e00224-20. [Google Scholar] [CrossRef]
- Afshari, R.; Akhavan, O.; Hamblin, M.R.; Varma, R.S. Review of Oxygenation with Nanobubbles: Possible Treatment for Hypoxic COVID-19 Patients. ACS Appl. Nano. Mater. 2021, 4, 11386–11412. [Google Scholar] [CrossRef]
- Rodríguez, S.M.; Richter, C.; Gálvez, J.B.; Vincent, M. Photocatalytic Degradation of Industrial Residual Waters. Sol. Energy 1996, 56, 401–410. [Google Scholar] [CrossRef]
- Gümüş, D.; Akbal, F. Photocatalytic Degradation of Textile Dye and Wastewater. Water. Air. Soil Pollut. 2011, 216, 117–124. [Google Scholar] [CrossRef]
- Khan, S.H.; Pathak, B. Zinc Oxide Based Photocatalytic Degradation of Persistent Pesticides: A Comprehensive Review. Environ. Nanotechnol. Monit. Manag. 2020, 13, 100290. [Google Scholar] [CrossRef]
- Fakhri, A.; Naji, M. Degradation Photocatalysis of Tetrodotoxin as a Poison by Gold Doped PdO Nanoparticles Supported on Reduced Graphene Oxide Nanocomposites and Evaluation of Its Antibacterial Activity. J. Photochem. Photobiol. Biol. 2017, 167, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Ziolli, R.L.; Jardim, W.F. Photocatalytic Decomposition of Seawater-Soluble Crude-Oil Fractions Using High Surface Area Colloid Nanoparticles of TiO2. J. Photochem. Photobiol. Chem. 2002, 147, 205–212. [Google Scholar] [CrossRef]
- Rachna; Rani, M.; Shanker, U. Degradation of Tricyclic Polyaromatic Hydrocarbons in Water, Soil and River Sediment with a Novel TiO2 Based Heterogeneous Nanocomposite. J. Environ. Manag. 2019, 248, 109340. [Google Scholar] [CrossRef] [PubMed]
- Meidanchi, A.; Akhavan, O.; Khoei, S.; Shokri, A.A.; Hajikarimi, Z.; Khansari, N. ZnFe2O4 Nanoparticles as Radiosensitizers in Radiotherapy of Human Prostate Cancer Cells. Mater. Sci. Eng. 2015, 46, 394–399. [Google Scholar] [CrossRef] [PubMed]
- Akhavan, O.; Ghaderi, E. Escherichia coli bacteria reduce graphene oxide to bactericidal graphene in a self-limiting manner. Carbon 2012, 50, 1853–1860. [Google Scholar] [CrossRef]
- Ren, S.; Boo, C.; Guo, N.; Wang, S.; Elimelech, M.; Wang, Y. Photocatalytic Reactive Ultrafiltration Membrane for Removal of Antibiotic Resistant Bacteria and Antibiotic Resistance Genes from Wastewater Effluent. Environ. Sci. Technol. 2018, 52, 8666–8673. [Google Scholar] [CrossRef]
- Mali, S.C.; Dhaka, A.; Githala, C.K.; Trivedi, R. Green Synthesis of Copper Nanoparticles Using Celastrus Paniculatus Willd. Leaf Extract and Their Photocatalytic and Antifungal Properties. Biotechnol. Rep. 2020, 27, e00518. [Google Scholar] [CrossRef]
- Akhavan, O.; Choobtashani, M.; Ghaderi, E. Protein Degradation and RNA Efflux of Viruses Photocatalyzed by Graphene-Tungsten Oxide Composite under Visible Light Irradiation. J. Phys. Chem. 2012, 116, 9653–9659. [Google Scholar] [CrossRef]
- Ebrahimi, M.; Asadi, M.; Akhavan, O. Graphene-Based Nanomaterials in Fighting the Most Challenging Viruses and Immunogenic Disorders. ACS Biomater. Sci. Eng. 2022, 8, 54–81. [Google Scholar] [CrossRef]
- Akhavan, O.; Ghaderi, E.; Rahimi, K. Adverse Effects of Graphene Incorporated in TiO2 Photocatalyst on Minuscule Animals under Solar Light Irradiation. J. Mater. Chem. 2012, 22, 23260–23266. [Google Scholar] [CrossRef]
- Sarkar, S.; Chakraborty, S.; Bhattacharjee, C. Photocatalytic Degradation of Pharmaceutical Wastes by Alginate Supported TiO2 Nanoparticles in Packed Bed Photo Reactor (PBPR). Ecotoxicol. Environ. Saf. 2015, 2, 35. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Zhou, S.; Yang, J.; Wang, H.; Yu, H.; Chen, H.; Zhao, Y.; Yuan, X.; Chu, W.; Li, H. Near-Infrared Light Responsive TiO2 for Efficient Solar Energy Utilization. Adv. Funct. Mater. 2022, 32, 2108977. [Google Scholar] [CrossRef]
- Hunge, Y.M.; Yadav, A.A.; Khan, S.; Takagi, K.; Suzuki, N.; Teshima, K.; Terashima, C.; Fujishima, A. Photocatalytic Degradation of Bisphenol A Using Titanium Dioxide@nanodiamond Composites under UV Light Illumination. J. Colloid Interf. Sci. 2021, 582, 1058–1066. [Google Scholar] [CrossRef] [PubMed]
- Hunge, Y.M.; Yadav, A.A.; Kang, S.W.; Kim, H. Photocatalytic Degradation of Tetracycline Antibiotics Using Hydrothermally Synthesized Two-Dimensional Molybdenum Disulfide/Titanium Dioxide Composites. J. Colloid Interf. Sci. 2022, 606, 454–463. [Google Scholar] [CrossRef]
- Varma, K.S.; Tayade, R.J.; Shah, K.J.; Joshi, P.A.; Shukla, A.D.; Gandhi, V.G. Photocatalytic Degradation of Pharmaceutical and Pesticide Compounds (PPCs) Using Doped TiO2 Nanomaterials: A Review. Water Energ. Nexus 2020, 3, 46–61. [Google Scholar] [CrossRef]
- Rokhsat, E.; Akhavan, O. Improving the Photocatalytic Activity of Graphene Oxide/ZnO Nanorod Films by UV Irradiation. Appl. Surf. Sci. 2016, 371, 590–595. [Google Scholar] [CrossRef]
- Akhavan, O. Thickness Dependent Activity of Nanostructured TiO2/α- Fe2O3 Photocatalyst Thin Films. Appl. Surf. Sci. 2010, 257, 1724–1728. [Google Scholar] [CrossRef]
- Begum, S.; Ahmaruzzaman, M. Biogenic Synthesis of SnO2/Activated Carbon Nanocomposite and Its Application as Photocatalyst in the Degradation of Naproxen. Appl. Surf. Sci. 2018, 449, 780–789. [Google Scholar] [CrossRef]
- Akhavan, O.; Ghaderi, E. Cu and CuO Nanoparticles Immobilized by Silica Thin Films as Antibacterial Materials and Photocatalysts. Surf. Coat. Technol. 2010, 205, 219–223. [Google Scholar] [CrossRef]
- Chaudhari, S.M.; Gonsalves, O.S.; Nemade, P.R. Enhanced Photocatalytic Degradation of Diclofenac with Agl/CeO2: A Comparison with Mn, Cu and Ag-Doped CeO2. Mater. Res. Bull. 2021, 143, 111463. [Google Scholar] [CrossRef]
- Zirak, M.; Akhavan, O.; Moradlou, O.; Nien, Y.T.; Moshfegh, A.Z. Vertically Aligned ZnO@CdS Nanorod Heterostructures for Visible Light Photoinactivation of Bacteria. J. Alloys Compd. 2014, 590, 507–513. [Google Scholar] [CrossRef]
- Czech, B.; Zygmunt, P.; Kadirova, Z.C.; Yubuta, K.; Hojamberdiev, M. Effective Photocatalytic Removal of Selected Pharmaceuticals and Personal Care Products by Elsmoreite/Tungsten Oxide@ZnS Photocatalyst. J. Environ. Manag. 2020, 270, 110870. [Google Scholar] [CrossRef]
- Yadav, A.A.; Hunge, Y.M.; Kang, S.W. Porous Nanoplate-like Tungsten Trioxide/Reduced Graphene Oxide Catalyst for Sonocatalytic Degradation and Photocatalytic Hydrogen Production. Surf. Interfaces 2021, 24, 101075. [Google Scholar] [CrossRef]
- Wang, Y.; Niu, J.; Zhang, Z.; Long, X. Sono-Photocatalytic Degradation of Organic Pollutants in Water. Prog. Chem. 2008, 20, 1621–1627. [Google Scholar] [CrossRef] [Green Version]
- Akhavan, O. Lasting Antibacterial Activities of Ag-TiO2/Ag/a-TiO2 Nanocomposite Thin Film Photocatalysts under Solar Light Irradiation. J. Colloid Interf. Sci. 2009, 336, 117–124. [Google Scholar] [CrossRef]
- Jiang, L.; Yang, J.; Zhou, S.; Yu, H.; Liang, J.; Chu, W.; Li, H.; Wang, H.; Wu, Z.; Yuan, X. Strategies to Extend Near-Infrared Light Harvest of Polymer Carbon Nitride Photocatalysts. Coord. Chem. Rev. 2021, 439, 213947. [Google Scholar] [CrossRef]
- Moma, J.; Baloyi, J. Modified Titanium Dioxide for Photocatalytic Applications. In Photocatalysts-Applications and Attributes; BahadarKhab, S., Akhtar, K., Eds.; ItechOpen: London, UK, 2019. [Google Scholar]
- Hojamberdiev, M.; Czech, B.; Göktaş, A.C.; Yubuta, K.; Kadirova, Z.C. SnO2@ZnS Photocatalyst with Enhanced Photocatalytic Activity for the Degradation of Selected Pharmaceuticals and Personal Care Products in Model Wastewater. J. Alloys Compd. 2020, 827, 154339. [Google Scholar] [CrossRef]
- Ahmadpour, N.; Sayadi, M.H.; Sobhani, S.; Hajiani, M. Photocatalytic Degradation of Model Pharmaceutical Pollutant by Novel Magnetic TiO2@ZnFe2O4/Pd Nanocomposite with Enhanced Photocatalytic Activity and Stability under Solar Light Irradiation. J. Environ. Manag. 2020, 271, 110964. [Google Scholar] [CrossRef]
- Zhang, Q.; Peng, Y.; Deng, F.; Wang, M.; Chen, D. Porous Z-Scheme MnO2/Mn-Modified Alkalinized g-C3N4 Heterojunction with Excellent Fenton-like Photocatalytic Activity for Efficient Degradation of Pharmaceutical Pollutants. Sep. Purif. Technol. 2020, 246, 116890. [Google Scholar] [CrossRef]
- Jouyandeh, M.; Tavakoli, O.; Sarkhanpour, R.; Sajadi, S.M.; Zarrintaj, P.; Rabiee, N.; Akhavan, O.; Lima, E.C.; Saeb, M.R. Green Products from Herbal Medicine Wastes by Subcritical Water Treatment. J. Hazard. Mater. 2022, 424, 127294. [Google Scholar] [CrossRef]
- Cregg, R.; Russo, G.; Gubbay, A.; Branford, R.; Sato, H. Pharmacogenetics of Analgesic Drugs. Br. J. Pain 2013, 7, 2049463713507439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Rensburg, R.; Reuter, H. An Overview of Analgesics: Nsaids, Paracetamol, and Topical Analgesics Part 1. S. Afr. Fam. Pract. 2019, 61, S4–S10. [Google Scholar] [CrossRef]
- Wu, S.; Zhang, L.; Chen, J. Paracetamol in the Environment and Its Degradation by Microorganisms. Appl. Microbiol. Biotechnol. 2012, 96, 875–884. [Google Scholar] [CrossRef] [PubMed]
- Acetaminophen (Tylenol): A Pain to the Environment! CHEM 331: Environmental Organic Chemistry March 20, 2008. Available online: https://web.viu.ca/krogh/chem331/Acetaminophen.pdf (accessed on 12 May 2022).
- Dalida, M.L.P.; Amer, K.M.S.; Su, C.C.; Lu, M.C. Photocatalytic Degradation of Acetaminophen in Modified TiO2 under Visible Irradiation. Environ. Sci. Pollut. Res. 2014, 21, 1208–1216. [Google Scholar] [CrossRef] [PubMed]
- Lozano-Morales, S.A.; Morales, G.; López Zavala, M.Á.; Arce-Sarria, A.; Machuca-Martínez, F. Photocatalytic Treatment of Paracetamol Using TiO2 Nanotubes: Effect of PH. Processes 2019, 7, 319. [Google Scholar] [CrossRef] [Green Version]
- Jayasree, P.; Remya, N. Photocatalytic Degradation of Paracetamol Using Aluminosilicate Supported TiO2. Water Sci. Technol. 2020, 82, 2114–2124. [Google Scholar] [CrossRef]
- Chang, C.T.; Wang, J.J.; Ouyang, T.; Zhang, Q.; Jing, Y.H. Photocatalytic Degradation of Acetaminophen in Aqueous Solutions by TiO2/ZSM-5 Zeolite with Low Energy Irradiation. Mater. Sci. Eng. Solid State Mater. Adv. Technol. 2015, 196, 53–60. [Google Scholar] [CrossRef]
- Jallouli, N.; Elghniji, K.; Trabelsi, H.; Ksibi, M. Photocatalytic Degradation of Paracetamol on TiO2 Nanoparticles and TiO2/Cellulosic Fiber under UV and Sunlight Irradiation. Arab. J. Chem. 2017, 10, S3640–S3645. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, T.A.; Mendo, S.G.; Ferreira, L.P.; Neng, N.R.; Oliveira, M.C.; Gil, A.; Carvalho, M.D.; Monteiro, O.C.; Nogueira, J.M.F.; Calhorda, M.J. Photocatalytic Degradation of Acetaminophen and Caffeine Using Magnetite–Hematite Combined Nanoparticles: Kinetics and Mechanisms. Environ. Sci. Pollut. Res. 2021, 28, 17228–17243. [Google Scholar] [CrossRef]
- Al Marzouqi, F.; Selvaraj, R.; Kim, Y. Rapid Photocatalytic Degradation of Acetaminophen and Levofloxacin Using G-C3N4 Nanosheets under Solar Light Irradiation. Mater. Res. Express 2019, 6, 125538. [Google Scholar] [CrossRef]
- Fan, G.; Peng, H.; Zhang, J.; Zheng, X.; Zhu, G.; Wang, S.; Hong, L. Degradation of Acetaminophen in Aqueous Solution under Visible Light Irradiation by Bi-Modified Titanate Nanomaterials: Morphology Effect, Kinetics and Mechanism. Catal. Sci. Technol. 2018, 8, 5906–5919. [Google Scholar] [CrossRef]
- Sharma, K.; Kaushik, G. NSAIDS in the Environment: From Emerging Problem to Green Solution OPEN ACCESS. Ann. Pharmacol. Pharm. 2017, 2, 1077. [Google Scholar]
- Tyumina, E.A.; Bazhutin, G.A.; Cartagena Gómez, A.D.P.; Ivshina, I.B. Nonsteroidal Anti-Inflammatory Drugs as Emerging Contaminants. Microbiol. Russian Fed. 2020, 89, 148–163. [Google Scholar] [CrossRef]
- Karimi, P.; Baneshi, M.M.; Malakootian, M. Photocatalytic Degradation of Aspirin from Aqueous Solutions Using the UV/ZnO Process: Modelling, Analysis and Optimization by Response Surface Methodology (RSM). Desalin. Water Treat. 2019, 161, 354–364. [Google Scholar] [CrossRef] [Green Version]
- Mukherjee, D.; Ray, A.K.; Barghi, S. Mechanism of Acetyl Salicylic Acid (Aspirin) Degradation under Solar Light in Presence of a TiO2-Polymeric Film Photocatalyst. Processes 2016, 4, 13. [Google Scholar] [CrossRef]
- Bianchi, C.L.; Sacchi, B.; Pirola, C.; Demartin, F.; Cerrato, G.; Morandi, S.; Capucci, V. Aspirin and Paracetamol Removal Using a Commercial Micro-Sized TiO2 Catalyst in Deionized and Tap Water. Environ. Sci. Pollut. Res. 2017, 24, 12646–12654. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Ma, Q.; Wang, S.; Song, S.; Li, B.; Guo, R.; Cheng, X.; Cheng, Q. Photocatalytic Performance and Degradation Mechanism of Aspirin by TiO2 through Response Surface Methodology. Catalysts 2018, 8, 118. [Google Scholar] [CrossRef] [Green Version]
- Moore, N.; Carleton, B.; Blin, P.; Bosco-Levy, P.; Droz, C. Does Ibuprofen Worsen COVID-19? Drug Saf. 2020, 43, 611–614. [Google Scholar] [CrossRef]
- Tadjarodi, A.; Akhavan, O.; Bijanzad, K.; Khiavi, M.M. Mechanochemically Prepared BiOCl Nanoplates for Removal of Rhodamine B and Pentachlorophenol. Monatshefte Chemie 2016, 147, 685–696. [Google Scholar] [CrossRef]
- Arthur, R.B.; Bonin, J.L.; Ardill, L.P.; Rourk, E.J.; Patterson, H.H.; Stemmler, E.A. Photocatalytic Degradation of Ibuprofen over BiOCl Nanosheets with Identification of Intermediates. J. Hazard. Mater. 2018, 358, 1–9. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, Z.; Yu, J.C. Photocatalytic Degradation of Ibuprofen on S-Doped BiOBr. Chemosphere 2021, 278, 130376. [Google Scholar] [CrossRef] [PubMed]
- Suarez-Escobar, A.F.; Conde-Rivera, L.R.; Lopez-Suarez, F.E.; Illán-Gómez, M.J.; Gonzalez-Hernandez, K.S.; Chalapud-Morales, J.S. Heterogeneous Photocatalytic Degradation of Ibuprofen Over TiO2–Ag Supported on Activated Carbon from Waste Tire Rubber. Top. Catal. 2021, 64, 51–64. [Google Scholar] [CrossRef]
- Romeiro, A.; Azenha, M.E.; Canle, M.; Rodrigues, V.H.N.; Da Silva, J.P.; Burrows, H.D. Titanium Dioxide Nanoparticle Photocatalysed Degradation of Ibuprofen and Naproxen in Water: Competing Hydroxyl Radical Attack and Oxidative Decarboxylation by Semiconductor Holes. ChemistrySelect 2018, 3, 10915–10924. [Google Scholar] [CrossRef]
- Pelosato, R.; Carrara, V.; Sora, I.N. Enhanced Photocatalytic Degradation of Ibuprofen in Aqueous Solution under Visible-Light Irradiation: Effects of LaFeO3 and Cu/LaFeO3. Chem. Eng. Trans. 2019, 73, 181–186. [Google Scholar] [CrossRef]
- Jallouli, N.; Pastrana-Martínez, L.M.; Ribeiro, A.R.; Moreira, N.F.F.; Faria, J.L.; Hentati, O.; Silva, A.M.T.; Ksibi, M. Heterogeneous Photocatalytic Degradation of Ibuprofen in Ultrapure Water, Municipal and Pharmaceutical Industry Wastewaters Using a TiO2/UV-LED System. Chem. Eng. J. 2018, 334, 976–984. [Google Scholar] [CrossRef]
- Tanveer, M.; Guyer, G.T.; Abbas, G. Photocatalytic Degradation of Ibuprofen in Water Using TiO2 and ZnO under Artificial UV and Solar Irradiation. Water Environ. Res. 2019, 91, 822–829. [Google Scholar] [CrossRef]
- Shafeei, N.; Asadollahfardi, G.; Moussavi, G.; Akbar Boojar, M.M. Degradation of Ibuprofen in the Photocatalytic Process with Doped TiO2 as Catalyst and UVA-LED as Existing Source. Desalin. Water Treat. 2019, 142, 341–352. [Google Scholar] [CrossRef]
- Rastkari, N.; Eslami, A.; Nasseri, S.; Piroti, E.; Asadi, A. Optimizing Parameters on Nanophotocatalytic Degradation of Ibuprofen Using UVC/ZnO Processes by Response Surface Methodology. Polish J. Environ. Stud. 2017, 26, 785–794. [Google Scholar] [CrossRef]
- Mohamed, A.; Salama, A.; Nasser, W.S.; Uheida, A. Photodegradation of Ibuprofen, Cetirizine, and Naproxen by PAN-MWCNT/TiO2–NH2 Nanofiber Membrane under UV Light Irradiation. Environ. Sci. Eur. 2018, 30, 1–9. [Google Scholar] [CrossRef]
- Khalaf, S.; Shoqeir, J.H.; Lelario, F.; Scrano, L.; Bufo, S.A.; Karaman, R. TiO2 and Active Coated Glass Photodegradation of Ibuprofen. Catalysts 2020, 10, 560. [Google Scholar] [CrossRef]
- Jiménez-Salcedo, M.; Monge, M.; Tena, M.T. Photocatalytic Degradation of Ibuprofen in Water Using TiO2/UV and g-C3N4/Visible Light: Study of Intermediate Degradation Products by Liquid Chromatography Coupled to High-Resolution Mass Spectrometry. Chemosphere 2019, 215, 605–618. [Google Scholar] [CrossRef] [PubMed]
- Simon, L.S. Biologic Effects of Nonsteroidal Anti-Inflammatory Drugs. Curr. Opin. Rheumatol. 1997, 9, 178–182. [Google Scholar] [CrossRef] [PubMed]
- Ong, C.K.S.; Lirk, P.; Tan, C.H.; Seymour, R.A. An Evidence-Based Update on Nonsteroidal Anti-Inflammatory Drugs. Clin. Med. Res. 2007, 5, 19–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wojcieszyńska, D.; Guzik, U. Naproxen in the Environment: Its Occurrence, Toxicity to Nontarget Organisms and Biodegradation. Appl. Microbiol. Biotechnol. 2020, 104, 1849–1857. [Google Scholar] [CrossRef] [Green Version]
- Štrbac, D.; Aggelopoulos, C.A.; Štrbac, G.; Dimitropoulos, M.; Novaković, M.; Ivetić, T.; Yannopoulos, S.N. Photocatalytic Degradation of Naproxen and Methylene Blue: Comparison between ZnO, TiO2 and Their Mixture. Proc. Saf. Environ. Prot. 2018, 113, 174–183. [Google Scholar] [CrossRef]
- Li, Z.; Liu, G.; Su, Q.; Lv, C.; Jin, X.; Wen, X. UV-Induced Photodegradation of Naproxen Using a Nano γ-FeOOH Composite: Degradation Kinetics and Photocatalytic Mechanism. Front. Chem. 2019, 7, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Fan, G.; Zhan, J.; Luo, J.; Zhang, J.; Chen, Z.; You, Y. Photocatalytic Degradation of Naproxen by a H2O2-Modified Titanate Nanomaterial under Visible Light Irradiation. Catal. Sci. Technol. 2019, 9, 4614–4628. [Google Scholar] [CrossRef]
- Kanakaraju, D.; Motti, C.A.; Glass, B.D.; Oelgemöller, M. Solar Photolysis versus TiO2-Mediated Solar Photocatalysis: A Kinetic Study of the Degradation of Naproxen and Diclofenac in Various Water Matrices. Environ. Sci. Pollut. Res. 2016, 23, 17437–17448. [Google Scholar] [CrossRef]
- Jallouli, N.; Elghniji, K.; Hentati, O.; Ribeiro, A.R.; Silva, A.M.T.; Ksibi, M. UV and Solar Photo-Degradation of Naproxen: TiO2 Catalyst Effect, Reaction Kinetics, Products Identification and Toxicity Assessment. J. Hazard. Mater. 2016, 304, 329–336. [Google Scholar] [CrossRef]
- Karimi, P.; Malakootian, M. Optimization of Photocatalytic Degradation of Naproxen from Aqueous Solutions with UV/ZnO Process: Response Surface Methodology (RSM). Glob. Nest J. 2020, 22, 369–380. [Google Scholar] [CrossRef]
- Fan, G.; Ning, R.; Luo, J.; Zhang, J.; Hua, P.; Guo, Y.; Li, Z. Visible-Light-Driven Photocatalytic Degradation of Naproxen by Bi-Modified Titanate Nanobulks: Synthesis, Degradation Pathway and Mechanism. J. Photochem. Photobiol. Chem. 2020, 386, 112108. [Google Scholar] [CrossRef]
- Fu, K.; Pan, Y.; Ding, C.; Shi, J.; Deng, H. Reduced Graphene Oxide/ZnIn2S4 Nanocomposite Photocatalyst with Enhanced Photocatalytic Performance for the Degradation of Naproxen under Visible Light Irradiation. Catalysts 2020, 10, 710. [Google Scholar] [CrossRef]
- Gupta, R.; Wadhwa, R. Mucolytic Medications; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Wilkinson, M.; Sugumar, K.; Milan, S.J.; Hart, A.; Crockett, A.; Crossingham, I. Mucolytics for Bronchiectasis. Cochrane Database Syst. Rev. 2014, 2014, 1289. [Google Scholar] [CrossRef] [PubMed]
- Olaleye, O.A.; Kaur, M.; Onyenaka, C.C. Ambroxol Hydrochloride Inhibits the Interaction between Severe Acute Respiratory Syndrome Coronavirus 2 Spike Protein’s Receptor Binding Domain and Recombinant Human ACE2. bioRxiv Prepr. Serv. Biol. 2020. [Google Scholar] [CrossRef]
- Tab, A.; Dahmane, M.; Belabed, C.; Bellal, B.; Richard, C.; Trari, M. High Efficiency Photocatalytic Degradation of Ambroxol over Mn Doped TiO2: Experimental Designs, Identification of Transformation Products, Mineralization and Mechanism. Sci. Total Environ. 2021, 780, 146451. [Google Scholar] [CrossRef]
- Bielen, A.; Šimatović, A.; Kosić-Vukšić, J.; Senta, I.; Ahel, M.; Babić, S.; Jurina, T.; González Plaza, J.J.; Milaković, M.; Udiković-Kolić, N. Negative Environmental Impacts of Antibiotic-Contaminated Effluents from Pharmaceutical Industries. Water Res. 2017, 126, 79–87. [Google Scholar] [CrossRef]
- Polianciuc, S.I.; Gurzău, A.E.; Kiss, B.; Georgia Ștefan, M.; Loghin, F. Antibiotics in the Environment: Causes and Consequences. Med. Pharm. Rep. 2020, 93, 231. [Google Scholar] [CrossRef]
- Čizmić, M.; Ljubas, D.; Rožman, M.; Ašperger, D.; Ćurković, L.; Babíc, S. Photocatalytic Degradation of Azithromycin by Nanostructured TiO2 Film: Kinetics, Degradation Products, and Toxicity. Materials 2019, 16, 873. [Google Scholar] [CrossRef] [Green Version]
- Luo, X.; Hao, T.; Yue, L.; Hong, G.; Lu, Y. Azithromycin Wastewater Treatment with La Doping Titanium Dioxide /Active Carbon Composites; Atlantis Press: Amsterdam, The Netherlands, 2016; pp. 861–870. [Google Scholar] [CrossRef]
- Akhavan, O.; Ghaderi, E. Photocatalytic Reduction of Graphene Oxide Nanosheets on TiO2 Thin Film for Photoinactivation of Bacteria in Solar Light Irradiation. J. Phys. Chem. 2009, 113, 20214–20220. [Google Scholar] [CrossRef]
- Naraginti, S.; Yu, Y.Y.; Fang, Z.; Yong, Y.C. Visible Light Degradation of Macrolide Antibiotic Azithromycin by Novel ZrO2/Ag@TiO2 Nanorod Composite: Transformation Pathways and Toxicity Evaluation. Proc. Saf. Environ. Prot. 2019, 125, 39–49. [Google Scholar] [CrossRef]
- Ospino-Atehortúa, B.A.; Zúñiga-Benítez, H.; Peñuela, G.A. Potential Application of Persulfate and Simulated Sunlight Radiation on Azithromycin Removal. Environ. Eng. Res. 2021, 26, 200189. [Google Scholar] [CrossRef]
- Li, C.; Jin, H.; Hou, Z.; Guo, Y. Study on Degradation of Azithromycin Antibiotics by Molybdenum Sulfide Graphene Oxide Composites under Visible Light. IOP Conf. Ser. Mater. Sci. Eng. 2020, 774, 012019. [Google Scholar] [CrossRef]
- Sayadi, M.H.; Sobhani, S.; Shekari, H. Photocatalytic Degradation of Azithromycin Using GO@Fe3O4/ ZnO/ SnO2 Nanocomposites. J. Clean. Prod. 2019, 232, 127–136. [Google Scholar] [CrossRef]
- Pham, T.D.M.; Ziora, Z.M.; Blaskovich, M.A.T. Quinolone Antibiotics. Medchemcomm 2019, 10, 1719–1739. [Google Scholar] [CrossRef]
- Sirtori, C.; Zapata, A.; Malato, S.; Gernjak, W.; Fernández-Alba, A.R.; Agüera, A. Solar Photocatalytic Treatment of Quinolones: Intermediates and Toxicity Evaluation. Photochem. Photobiol. Sci. 2009, 8, 644–651. [Google Scholar] [CrossRef]
- Song, W.; Muste, J.C.; Greenlee, T.E.; Singh, R.P. Chloroquine and Hydroxychloroquine Toxicity. Am. J. Ophthalm. Clin. Trials 2020, 129, 1506–1507. [Google Scholar] [CrossRef]
- Gasmi, A.; Peana, M.; Noor, S.; Lysiuk, R.; Menzel, A.; Gasmi Benahmed, A.; Bjørklund, G. Chloroquine and Hydroxychloroquine in the Treatment of COVID-19: The Never-Ending Story. Appl. Microbiol. Biotechnol. 2021, 105, 1333–1343. [Google Scholar] [CrossRef]
- da Silva, P.L.; Nippes, R.P.; Macruz, P.D.; Hegeto, F.L.; Olsen Scaliante, M.H.N. Photocatalytic Degradation of Hydroxychloroquine Using ZnO Supported on Clinoptilolite Zeolite. Water Sci. Technol. 2021, 84, 763–776. [Google Scholar] [CrossRef]
- Bensalah, N.; Midassi, S.; Ahmad, M.I.; Bedoui, A. Degradation of Hydroxychloroquine by Electrochemical Advanced Oxidation Processes. Chem. Eng. J. 2020, 402, 126279. [Google Scholar] [CrossRef]
- Yi, X.H.; Ji, H.; Wang, C.C.; Li, Y.; Li, Y.H.; Zhao, C.; Wang, A.; Fu, H.; Wang, P.; Zhao, X.; et al. Photocatalysis-Activated SR-AOP over PDINH/MIL-88A(Fe) Composites for Boosted Chloroquine Phosphate Degradation: Performance, Mechanism, Pathway and DFT Calculations. Appl. Catal. Environ. 2021, 293, 120229. [Google Scholar] [CrossRef]
- Barnes, P.J. Anti-Inflammatory Actions of Glucocorticoids: Molecular Mechanisms. Clin. Sci. 1998, 94, 557–572. [Google Scholar] [CrossRef] [Green Version]
- Dexamethasone in Hospitalized Patients with Covid-19. N. Engl. J. Med. 2021. [CrossRef]
- Vardy, J.; Chiew, K.S.; Galica, J.; Pond, G.R.; Tannock, I.F. Side Effects Associated with the Use of Dexamethasone for Prophylaxis of Delayed Emesis after Moderately Emetogenic Chemotherapy. Br. J. Cancer 2006, 94, 1011–1015. [Google Scholar] [CrossRef]
- Pazoki, M.; Parsa, M.; Farhadpour, R. Removal of the Hormones Dexamethasone (DXM) by Ag Doped on TiO2 Photocatalysis. J. Environ. Chem. Eng. 2016, 4, 4426–4434. [Google Scholar] [CrossRef]
- Ghenaatgar, A.; Tehrani, R.M.A.; Khadir, A. Photocatalytic Degradation and Mineralization of Dexamethasone Using WO3 and ZrO2 Nanoparticles: Optimization of Operational Parameters and Kinetic Studies. J. Water Process Eng. 2019, 32, 100969. [Google Scholar] [CrossRef]
- da Silva, W.L.; Lansarin, M.A.; dos Santos, J.H.Z.; Da Rocha, Z.N.; Pepe, I.M. Electrochemical and Catalytic Studies of a Supported Photocatalyst Produced from Petrochemical Residue in the Photocatalytic Degradation of Dexamethasone and Guaifenesin Drugs. Water. Air. Soil Pollut. 2016, 227, 1–9. [Google Scholar] [CrossRef]
- Awwad, N.S.; El-Khalafawy, A.; Ibrahium, H.A.; Hamdy, M.S. Photocatalytic Degradation of Cortisone Acetate by Using Graphite Doped Ceria Nanoparticles under Visible Light Illumination. Mater. Res. Express 2019, 6, 095907. [Google Scholar] [CrossRef]
- Amani, H.; Habibey, R.; Hajmiresmail, S.J.; Latifi, S.; Pazoki-Toroudi, H.; Akhavan, O. Antioxidant Nanomaterials in Advanced Diagnoses and Treatments of Ischemia Reperfusion Injuries. J. Mater. Chem. 2017, 5, 9452–9476. [Google Scholar] [CrossRef] [PubMed]
- Amani, H.; Habibey, R.; Shokri, F.; Hajmiresmail, S.J.; Akhavan, O.; Mashaghi, A.; Pazoki-Toroudi, H. Selenium Nanoparticles for Targeted Stroke Therapy through Modulation of Inflammatory and Metabolic Signaling. Sci. Rep. 2019, 9, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Kosonen, J.; Kronberg, L. The Occurrence of Antihistamines in Sewage Waters and in Recipient Rivers. Environ. Sci. Pollut. Res. 2009, 16, 555–564. [Google Scholar] [CrossRef]
- Abdel-Raouf, N.; Al-Homaidan, A.A.; Ibraheem, I.B.M. Microalgae and Wastewater Treatment. Saudi J. Biol. Sci. 2012, 19, 257–275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gelotte, C.K.; Zimmerman, B.A.; Thompson, G.A. Single-Dose Pharmacokinetic Study of Diphenhydramine HCl in Children and Adolescents. Clin. Pharmacol. Drug Dev. 2018, 7, 400–407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, L.; Yi, C.; Wu, Q.; Li, Z.; Zhang, W.; Hong, H. Photocatalytic Degradation of Diphenhydramine in Aqueous Solution by Natural Dolomite. RSC Adv. 2020, 10, 38663–38671. [Google Scholar] [CrossRef] [PubMed]
- Pastrana-Martínez, L.M.; Faria, J.L.; Doña-Rodríguez, J.M.; Fernández-Rodríguez, C.; Silva, A.M.T. Degradation of Diphenhydramine Pharmaceutical in Aqueous Solutions by Using Two Highly Active TiO2 Photocatalysts: Operating Parameters and Photocatalytic Mechanism. Appl. Catal. Environ. 2012, 113–114, 221–227. [Google Scholar] [CrossRef]
- López, N.; Marco, P.; Giménez, J.; Esplugas, S. Photocatalytic Diphenhydramine Degradation under Different Radiation Sources: Kinetic Studies and Energetic Comparison. Appl. Catal. Environ. 2018, 220, 497–505. [Google Scholar] [CrossRef]
- Morales-Torres, S.; Pastrana-Martínez, L.M.; Figueiredo, J.L.; Faria, J.L.; Silva, A.M.T. Graphene Oxide-P25 Photocatalysts for Degradation of Diphenhydramine Pharmaceutical and Methyl Orange Dye. Appl. Surf. Sci. 2013, 275, 361–368. [Google Scholar] [CrossRef]
- Davari, N.; Farhadian, M.; Nazar, A.R.S.; Homayoonfal, M. Degradation of Diphenhydramine by the Photocatalysts of ZnO/Fe2O3 and TiO2/Fe2O3 Based on Clinoptilolite: Structural and Operational Comparison. J. Environ. Chem. Eng. 2017, 5, 5707–5720. [Google Scholar] [CrossRef]
- Arai, N.; Nakamizo, T.; Ihara, H.; Koide, T.; Nakamura, A.; Tabuse, M.; Miyazaki, H. Histamine H2-Blocker and Proton Pump Inhibitor Use and the Risk of Pneumonia in Acute Stroke: A Retrospective Analysis on Susceptible Patients. PLoS ONE 2017, 12, e0169300. [Google Scholar] [CrossRef]
- Vatti, S.K.; Gupta, S.; Raj, R.P.; Selvam, P. Periodic Mesoporous Titania with Anatase and Bronze Phases—The New Generation Photocatalyst: Synthesis, Characterisation, and Application in Environmental Remediation. New J. Chem. 2020, 44, 16269–16284. [Google Scholar] [CrossRef]
- Molla, M.A.I.; Tateishi, I.; Furukawa, M.; Katsumata, H.; Suzuki, T.; Kaneco, S. Photocatalytic Removal of Famotidine with TiO2 from Water in the Presence of Dye under Visible Light Irradiation. Desalin. Water Treat. 2017, 87, 338–347. [Google Scholar] [CrossRef]
- Keane, D.; Basha, S.; Nolan, K.; Morrissey, A.; Oelgemöller, M.; Tobin, J.M. Photodegradation of Famotidine by Integrated Photocatalytic Adsorbent (IPCA) and Kinetic Study. Catal. Lett. 2011, 141, 300–308. [Google Scholar] [CrossRef]
- Abongwa, M.; Martin, R.J.; Robertson, A.P. A Brief Review on the Mode of Action of Antinematodal Drugs. Acta Vet. Brno. 2017, 67, 137–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chalfie, M.; Girard, L. WormBook the Online Review of C. Elegans Biology. West Coast Worm Meet. 2007, 35, D472–D475. [Google Scholar]
- Chai, J.Y. Praziquantel Treatment in Trematode and Cestode Infections: An Update. Infect. Chemother. 2013, 45, 32–43. [Google Scholar] [CrossRef] [Green Version]
- Oyeyemi, O.T.; Okunlola, O.A.; Adebayo, A.D. Assessment of Schistosomiasis Endemicity and Preventive Treatment on Coronavirus Disease 2019 Outcomes in Africa. New Microb. New Infect. 2020, 38, 100821. [Google Scholar] [CrossRef]
- Čizmić, M.; Ljubas, D.; Ćurković, L.; Škorić, I.; Babić, S. Kinetics and Degradation Pathways of Photolytic and Photocatalytic Oxidation of the Anthelmintic Drug Praziquantel. J. Hazard. Mater. 2017, 323, 500–512. [Google Scholar] [CrossRef]
- Rossignol, J.F. Nitazoxanide: A First-in-Class Broad-Spectrum Antiviral Agent. Antiv. Res. 2014, 110, 94–103. [Google Scholar] [CrossRef] [Green Version]
- Bryant, A.; Lawrie, T.A.; Dowswell, T.; Fordham, E.J.; Mitchell, S.; Hill, S.R.; Tham, T.C. Ivermectin for Prevention and Treatment of COVID-19 Infection: A Systematic Review, Meta-Analysis, and Trial Sequential Analysis to Inform Clinical Guidelines. Am. J. Ther. 2021, 28, e434–e460. [Google Scholar] [CrossRef]
- Havlíková, L.; Šatínský, D.; Solich, P. Aspects of Decontamination of Ivermectin and Praziquantel from Environmental Waters Using Advanced Oxidation Technology. Chemosphere 2016, 144, 21–28. [Google Scholar] [CrossRef]
- Bosco, S.M.D.; Barbosa, I.M.; Candello, F.P.; Maniero, M.G.; Rath, S.; Guimaraes, J.R. Degradation of Ivermectin by Fenton and Photo-Fenton and Toxicity Test Using Daphnia Similis. J. Adv. Oxid. Technol. 2011. [Google Scholar] [CrossRef]
- Rafailidis, P.I.; Mavros, M.N.; Kapaskelis, A.; Falagas, M.E. Antiviral Treatment for Severe EBV Infections in Apparently Immunocompetent Patients. J. Clin. Virol. 2010, 49, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Wagstaff, A.J.; Faulds, D.; Goa, K.L. Aciclovir: A Reappraisal of Its Antiviral Activity, Pharmacokinetic Properties and Therapeutic Efficacy. Drugs 1994, 47, 153–205. [Google Scholar] [CrossRef] [PubMed]
- Russo, D.; Siciliano, A.; Guida, M.; Galdiero, E.; Amoresano, A.; Andreozzi, R.; Reis, N.M.; Li Puma, G.; Marotta, R. Photodegradation and Ecotoxicology of Acyclovir in Water under UV254 and UV254/H2O2 Processes. Water Res. 2017, 122, 591–602. [Google Scholar] [CrossRef] [Green Version]
- An, T.; An, J.; Gao, Y.; Li, G.; Fang, H.; Song, W. Photocatalytic Degradation and Mineralization Mechanism and Toxicity Assessment of Antivirus Drug Acyclovir: Experimental and Theoretical Studies. Appl. Catal. Environ. 2015, 164, 279–287. [Google Scholar] [CrossRef]
- Zhou, C.; Chen, J.; Xie, Q.; Wei, X.; Zhang, Y.N.; Fu, Z. Photolysis of Three Antiviral Drugs Acyclovir, Zidovudine and Lamivudine in Surface Freshwater and Seawater. Chemosphere 2015, 138, 792–797. [Google Scholar] [CrossRef] [PubMed]
- Jia, T.C.; Guo, J.T.; Wang, Z.; Zhu, X.S.; Zhang, Q.X.; Chen, P.; Yao, K.; Lv, W.Y.; Liu, G.G. Photodegradation Mechanisms of Acyclovir in Water and the Toxicity of Photoproducts. J. Radioanal. Nucl. Chem. 2019, 320, 823–830. [Google Scholar] [CrossRef]
- Alhumaid, S.; Al Mutair, A.; Al Alawi, Z.; Alhmeed, N.; Zaidi, A.R.Z.; Tobaiqy, M. Efficacy and Safety of Lopinavir/Ritonavir for Treatment of COVID-19: A Systematic Review and Meta-Analysis. Trop. Med. Infect. Dis. 2020, 5, 180. [Google Scholar] [CrossRef]
- Horby, P.W.; Mafham, M.; Bell, J.L.; Linsell, L.; Staplin, N.; Emberson, J.; Palfreeman, A.; Raw, J.; Elmahi, E.; Prudon, B.; et al. Lopinavir–Ritonavir in Patients Admitted to Hospital with COVID-19 (RECOVERY): A Randomised, Controlled, Open-Label, Platform Trial. Lancet 2020, 396, 1345–1352. [Google Scholar] [CrossRef]
- Bhavyasri, K.; Murali Balaram, V.; Nageswarao, R.; Rambabu, D.; Sasikiran Goud, E.; Ajitha, M. Development and Validation of Forced Degradation Studies of Atazanavir Using RP-HPLC and Characterization of Degradants by LC-MS/MS. Int. J. Pharm. Sci. Rev. Res. 2015, 33, 11–15. [Google Scholar]
- Donato, E.M.; Dias, C.L.; Rossi, R.C.; Valente, R.S.; Fröehlich, P.E.; Bergold, A.M. LC Method for Studies on the Stability of Lopinavir and Ritonavir in Soft Gelatin Capsules. Chromatographia 2006, 63, 437–443. [Google Scholar] [CrossRef]
- Agrawal, U.; Raju, R.; Udwadia, Z.F. Favipiravir: A New and Emerging Antiviral Option in COVID-19. Med. J. Armed Forces India 2020. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, T.; Honda, R. Electrochemical and Mechanistic Study of Oxidative Degradation of Favipiravir by Electrogenerated Superoxide through Proton-Coupled Electron Transfer. ACS Omega 2021, 6, 21730–21740. [Google Scholar] [CrossRef] [PubMed]
- Di Santo, N.; Ehrisman, J. Research Perspective: Potential Role of Nitazoxanide in Ovarian Cancer Treatment. Old Drug, New Purpose? Cancers 2013, 5, 1163–1176. [Google Scholar] [CrossRef] [PubMed]
- White, A.C. Nitazoxanide: A New Broad Spectrum Antiparasitic Agent. Expert Rev. Anti. Infect. Ther. 2004, 2, 43–49. [Google Scholar] [CrossRef]
- Zerón, H.M.; Calderón, J.M.; Coria, L.P.; Figueroa, J.M.; Vargas Contreras, M.J.; Aceves, H.L.V.; Salazar, F.M.C.; Hernández, D.C.; Vidaurri, E.M.; González, A.C.; et al. Nitazoxanide as an Early Treatment to Reduce the Intensity of COVID-19 Outbreaks among Health Personnel. World Acad. Sci. J. 2021, 3, 1–6. [Google Scholar] [CrossRef]
- Malesuik, M.D.; Gonçalves, H.M.L.; Paim, C.S.; Schapoval, E.E.S.; Steppe, M. LC: Analysis of Photodegradation Kinetics of Nitazoxanide in Pharmaceutical Formulations. J. Chromatogr. Sci. 2009, 47, 745–748. [Google Scholar] [CrossRef] [Green Version]
- Kokic, G.; Hillen, H.S.; Tegunov, D.; Dienemann, C.; Seitz, F.; Schmitzova, J.; Farnung, L.; Siewert, A.; Höbartner, C.; Cramer, P. Mechanism of SARS-CoV-2 Polymerase Stalling by Remdesivir. Nat. Commun. 2021, 12, 1–7. [Google Scholar] [CrossRef]
- Beigel, J.H.; Tomashek, K.M.; Dodd, L.E.; Mehta, A.K.; Zingman, B.S.; Kalil, A.C.; Hohmann, E.; Chu, H.Y.; Luetkemeyer, A.; Kline, S.; et al. Remdesivir for the Treatment of Covid-19—Final Report. N. Engl. J. Med. 2020, 383, 1813–1826. [Google Scholar] [CrossRef]
- Hamdy, M.M.A.; Abdel Moneim, M.M.; Kamal, M.F. Accelerated Stability Study of the Ester Prodrug Remdesivir: Recently FDA-Approved Covid-19 Antiviral Using Reversed-Phase-HPLC with Fluorimetric and Diode Array Detection. Biomed. Chromatogr. 2021, 35, e5212. [Google Scholar] [CrossRef] [PubMed]
- Vartak, R.; Patil, S.M.; Saraswat, A.; Patki, M.; Kunda, N.K.; Patel, K. Aerosolized Nanoliposomal Carrier of Remdesivir: An Effective Alternative for COVID-19 Treatment in Vitro. Nanomedicine 2021, 16, 1187–1202. [Google Scholar] [CrossRef]









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ebrahimi, M.; Akhavan, O. Nanomaterials for Photocatalytic Degradations of Analgesic, Mucolytic and Anti-Biotic/Viral/Inflammatory Drugs Widely Used in Controlling SARS-CoV-2. Catalysts 2022, 12, 667. https://doi.org/10.3390/catal12060667
Ebrahimi M, Akhavan O. Nanomaterials for Photocatalytic Degradations of Analgesic, Mucolytic and Anti-Biotic/Viral/Inflammatory Drugs Widely Used in Controlling SARS-CoV-2. Catalysts. 2022; 12(6):667. https://doi.org/10.3390/catal12060667
Chicago/Turabian StyleEbrahimi, Mahsa, and Omid Akhavan. 2022. "Nanomaterials for Photocatalytic Degradations of Analgesic, Mucolytic and Anti-Biotic/Viral/Inflammatory Drugs Widely Used in Controlling SARS-CoV-2" Catalysts 12, no. 6: 667. https://doi.org/10.3390/catal12060667