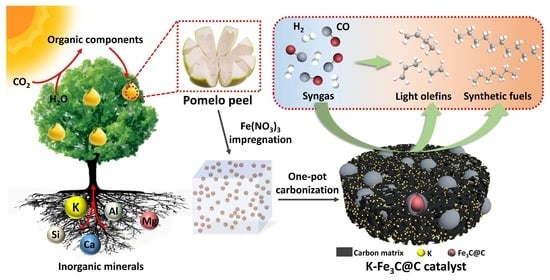
Direct Construction of K-Fe3C@C Nanohybrids Utilizing Waste Biomass of Pomelo Peel as High-Performance Fischer–Tropsch Catalysts
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of the Catalysts
2.2. Catalytic Performance of Fischer–Tropsch Synthesis
3. Materials and Methods
3.1. Materials
3.2. Catalyst Preparation
3.3. Catalyst Characterization
3.4. Fischer–Tropsch Synthesis Reaction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Davis, B.H. Fischer–Tropsch synthesis: Relationship between iron catalyst composition and process variables. Catal. Today 2003, 84, 83–98. [Google Scholar] [CrossRef]
- Li, J.; He, Y.; Tan, L.; Zhang, P.; Peng, X.; Oruganti, A.; Yang, G.; Abe, H.; Wang, Y.; Tsubaki, N. Integrated tuneable synthesis of liquid fuels via Fischer–Tropsch technology. Nat. Catal. 2018, 1, 787–793. [Google Scholar] [CrossRef]
- Teimouri, Z.; Abatzoglou, N.; Dalai, A.K. Kinetics and selectivity study of Fischer–Tropsch synthesis to C5+ hydrocarbons: A review. Catalysts 2021, 11, 330. [Google Scholar] [CrossRef]
- Chang, Q.; Zhang, C.; Liu, C.; Wei, Y.; Cheruvathur, A.V.; Dugulan, A.I.; Niemantsverdriet, J.W.; Liu, X.; He, Y.; Qing, M.; et al. Relationship between iron carbide phases (ε-Fe2C, Fe7C3, and χ-Fe5C2) and catalytic performances of Fe/SiO2 Fischer–Tropsch catalysts. ACS Catal. 2018, 8, 3304–3316. [Google Scholar] [CrossRef]
- Lin, Q.; Cheng, M.; Zhang, K.; Li, W.; Wu, P.; Chang, H.; Lv, Y.; Men, Z. Development of an iron-based Fischer–Tropsch catalyst with high attrition resistance and stability for industrial application. Catalysts 2021, 11, 908. [Google Scholar] [CrossRef]
- Li, S.; Yang, J.; Song, C.; Zhu, Q.; Xiao, D.; Ma, D. Iron carbides: Control synthesis and catalytic applications in COx hydrogenation and electrochemical HER. Adv. Mater. 2019, 31, 176–188. [Google Scholar] [CrossRef]
- Torres Galvis, H.M.; Bitter, J.H.; Khare, C.B.; Ruitenbeek, M.; Dugulan, A.I.; de Jong, K.P. Supported iron nanoparticles as catalysts for sustainable production of lower olefins. Science 2012, 335, 835–838. [Google Scholar] [CrossRef]
- Zhao, S.; Liu, X.-W.; Huo, C.-F.; Li, Y.-W.; Wang, J.; Jiao, H. Determining surface structure and stability of ε-Fe2C, χ-Fe5C2, θ-Fe3C and Fe4C phases under carburization environment from combined DFT and atomistic thermodynamic studies. Catal. Struct. React. 2014, 1, 44–60. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, H.; Liu, Y.; Ning, W.; Han, W.; Liu, H.; Huo, C. Preparation of iron carbides formed by iron oxalate carburization for Fischer–Tropsch synthesis. Catalysts 2019, 9, 347. [Google Scholar] [CrossRef]
- Zhuo, O.; Yang, L.; Gao, F.; Xu, B.; Wu, Q.; Fan, Y.; Zhang, Y.; Jiang, Y.; Huang, R.; Wang, X.; et al. Stabilizing the active phase of iron-based Fischer–Tropsch catalysts for lower olefins: Mechanism and strategy. Chem. Sci. 2019, 10, 6083–6090. [Google Scholar] [CrossRef]
- Ma, C.; Zhang, W.; Chang, Q.; Wang, X.; Wang, H.; Chen, H.; Wei, Y.; Zhang, C.; Xiang, H.; Yang, Y.; et al. θ-Fe3C dominated Fe@C core–shell catalysts for Fischer–Tropsch synthesis: Roles of θ-Fe3C and carbon shell. J. Catal. 2021, 393, 238–246. [Google Scholar] [CrossRef]
- Zhang, Q.; Gu, J.; Chen, J.; Qiu, S.; Wang, T. Facile fabrication of porous Fe@C nanohybrids from natural magnetite as excellent Fischer–Tropsch catalysts. Chem. Commun. 2020, 56, 4523–4526. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Xu, L.; Qian, J.; Bao, J.; Yan, C.; Li, H.; Li, H.; Zhang, S. Fe3C/Fe2O3 heterostructure embedded in N-doped graphene as a bifunctional catalyst for quasi-solid-state zinc-air batteries. Carbon 2019, 146, 763–771. [Google Scholar] [CrossRef]
- Xiong, H.; Moyo, M.; Motchelaho, M.A.; Tetana, Z.N.; Dube, S.M.A.; Jewell, L.L.; Coville, N.J. Fischer–Tropsch synthesis: Iron catalysts supported on N-doped carbon spheres prepared by chemical vapor deposition and hydrothermal approaches. J. Catal. 2014, 311, 80–87. [Google Scholar] [CrossRef]
- Ye, Z.; Zhang, P.; Lei, X.; Wang, X.; Zhao, N.; Yang, H. Iron carbides and nitrides: Ancient materials with novel prospects. Chem.-Eur. J. 2018, 24, 8922–8940. [Google Scholar] [CrossRef]
- Zhao, P.; Hua, X.; Xu, W.; Luo, W.; Chen, S.; Cheng, G. Metal–organic framework-derived hybrid of Fe3C nanorod-encapsulated, N-doped CNTs on porous carbon sheets for highly efficient oxygen reduction and water oxidation. Catal. Sci. Technol. 2016, 6, 6365–6371. [Google Scholar] [CrossRef]
- Arce-Saldaña, L.A.; Espinoza-Mosso, E.; Castro-Rodríguez, B.; Portillo-López, A.; Muñoz-Muñoz, F.; Dominguez, D.; Farías, M.H.; Soto, G. Plasma synthesis of carbon powder with embedded Fe3C nanoparticles for magnetic separation of biomolecules. Adv. Powder Technol. 2018, 29, 1035–1041. [Google Scholar] [CrossRef]
- Zhao, H.; Liu, J.; Yang, C.; Yao, S.; Su, H.; Gao, Z.; Dong, M.; Wang, J.; Hou, Y.; Li, W.; et al. Synthesis of iron-carbide nanoparticles: Identification of the active phase and mechanism of Fe-based Fischer–Tropsch synthesis. CCS Chem. 2021, 3, 2712–2724. [Google Scholar] [CrossRef]
- Yao, S.; Yang, C.; Zhao, H.; Li, S.; Lin, L.; Wen, W.; Liu, J.; Hu, G.; Li, W.; Hou, Y.; et al. Reconstruction of the wet chemical synthesis process: The case of Fe5C2 nanoparticles. J. Phys. Chem. C 2017, 121, 5154–5160. [Google Scholar] [CrossRef]
- De, S.; Balu, A.M.; van der Waal, J.C.; Luque, R. Biomass-derived porous carbon materials: Synthesis and catalytic applications. ChemCatChem 2015, 7, 1608–1629. [Google Scholar] [CrossRef]
- Tang, Z.E.; Lim, S.; Pang, Y.L.; Ong, H.C.; Lee, K.T. Synthesis of biomass as heterogeneous catalyst for application in biodiesel production: State of the art and fundamental review. Renew. Sustain. Energy Rev. 2018, 92, 235–253. [Google Scholar] [CrossRef]
- Wang, Z.; Shen, D.; Wu, C.; Gu, S. State-of-the-art on the production and application of carbon nanomaterials from biomass. Green Chem. 2018, 20, 5031–5057. [Google Scholar] [CrossRef]
- Qin, H.; Zhou, Y.; Bai, J.; Zhu, B.; Ni, Z.; Wang, L.; Liu, W.; Zhou, Q.; Li, X. Lignin-derived thin-walled graphitic carbon-encapsulated iron nanoparticles: Growth, characterization, and applications. ACS Sustain. Chem. Eng. 2017, 5, 1917–1923. [Google Scholar] [CrossRef]
- Schnepp, Z.; Wimbush, S.C.; Antonietti, M.; Giordano, C. Synthesis of highly magnetic iron carbide nanoparticles via a biopolymer route. Chem. Mater. 2010, 22, 5340–5344. [Google Scholar] [CrossRef]
- Xiao, L.; Ye, F.; Zhou, Y.; Zhao, G. Utilization of pomelo peels to manufacture value-added products: A review. Food Chem. 2021, 351, 129247–129261. [Google Scholar] [CrossRef]
- Oberoi, H.S.; Vadlani, P.V.; Nanjundaswamy, A.; Bansal, S.; Singh, S.; Kaur, S.; Babbar, N. Enhanced ethanol production from Kinnow mandarin (Citrus reticulata) waste via a statistically optimized simultaneous saccharification and fermentation process. Bioresour. Technol. 2011, 102, 1593–1601. [Google Scholar] [CrossRef]
- Yongvanich, N. Isolation of nanocellulose from pomelo fruit fibers by chemical treatments. J. Nat. Fibers 2015, 12, 323–331. [Google Scholar] [CrossRef]
- Zou, J.; Chai, W.; Liu, X.; Li, B.; Zhang, X.; Yin, T. Magnetic pomelo peel as a new absorption material for oil-polluted water. Desalin. Water Treat. 2015, 57, 12536–12545. [Google Scholar] [CrossRef]
- Alva, A.K.; Mattos, D.; Paramasivam, S.; Patil, B.; Dou, H.; Sajwan, K.S. Potassium management for optimizing citrus production and quality. Int. J. Fruit Sci. 2006, 6, 3–43. [Google Scholar] [CrossRef]
- Liang, Q.; Ye, L.; Huang, Z.H.; Xu, Q.; Bai, Y.; Kang, F.; Yang, Q.H. A honeycomb-like porous carbon derived from pomelo peel for use in high-performance supercapacitors. Nanoscale 2014, 6, 13831–13837. [Google Scholar] [CrossRef]
- Peng, H.; Ma, G.; Sun, K.; Zhang, Z.; Yang, Q.; Lei, Z. Nitrogen-doped interconnected carbon nanosheets from pomelo mesocarps for high performance supercapacitors. Electrochimica Acta 2016, 190, 862–871. [Google Scholar] [CrossRef]
- Peng, C.; Lang, J.; Xu, S.; Wang, X. Oxygen-enriched activated carbons from pomelo peel in high energy density supercapacitors. RSC Adv. 2014, 4, 54662–54667. [Google Scholar] [CrossRef]
- Zhao, R.; Wang, Z.; Yang, Y.; Xing, X.; Zou, T.; Wang, Z.; Hong, P.; Peng, S.; Wang, Y. Pd-functionalized SnO2 nanofibers prepared by shaddock peels as bio-templates for high gas sensing performance toward butane. Nanomaterials 2018, 9, 13–28. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; You, S.; Wang, W.; Liu, G.; Qi, D.; Chen, X.; Qu, J.; Ren, N. Biomass-derived porous Fe3C/tungsten carbide/graphitic carbon nanocomposite for efficient electrocatalysis of oxygen reduction. ACS Appl. Mater. Interfaces 2016, 8, 32307–32316. [Google Scholar] [CrossRef]
- Wang, C.; Liu, Y.; Cui, Z.; Yu, X.; Zhang, X.; Li, Y.; Zhang, Q.; Chen, L.; Ma, L. In situ synthesis of Cu nanoparticles on carbon for highly selective hydrogenation of furfural to furfuryl alcohol by using pomelo peel as the carbon source. ACS Sustain. Chem. Eng. 2020, 8, 12944–12955. [Google Scholar] [CrossRef]
- Zhao, S.; Wang, L.; Wang, Y.; Li, X. Hierarchically porous LaFeO3 perovskite prepared from the pomelo peel bio-template for catalytic oxidation of NO. J. Phys. Chem. Solids 2018, 116, 43–49. [Google Scholar] [CrossRef]
- Fan, G.; Jiang, Y.; Xin, J.; Zhang, Z.; Fu, X.; Xie, P.; Cheng, C.; Liu, Y.; Qu, Y.; Sun, K.; et al. Facile synthesis of Fe@Fe3C/C nanocomposites derived from bulrush for excellent electromagnetic wave-absorbing properties. ACS Sustain. Chem. Eng. 2019, 7, 18765–18774. [Google Scholar] [CrossRef]
- Cao, X.; Sun, S.; Sun, R. Application of biochar-based catalysts in biomass upgrading: A review. RSC Adv. 2017, 7, 48793–48805. [Google Scholar] [CrossRef]
- Han, Y.; Fang, C.; Ji, X.; Wei, J.; Ge, Q.; Sun, J. Interfacing with carbonaceous potassium promoters boosts catalytic CO2 hydrogenation of iron. ACS Catal. 2020, 10, 12098–12108. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, H.K.; Kim, K.; Rhim, G.B.; Youn, M.H.; Jeong, H.; Park, J.H.; Chun, D.H.; Kim, B.-H.; Park, J.C. Unravelling the K-promotion effect in highly active and stable Fe5C2 nanoparticles for catalytic linear α-olefin production. Mater. Adv. 2021, 2, 1050–1058. [Google Scholar] [CrossRef]
- Bai, J.; Qin, C.; Xu, Y.; Du, Y.; Ma, G.; Ding, M. Biosugarcane-based carbon support for high-performance iron-based Fischer–Tropsch synthesis. iScience 2021, 24, 102715–102721. [Google Scholar] [CrossRef] [PubMed]
- van Lith, S.C.; Jensen, P.A.; Frandsen, F.J.; Glarborg, P. Release to the gas phase of inorganic elements during wood combustion. part 2: Influence of fuel composition. Energy Fuel. 2008, 22, 1598–1609. [Google Scholar] [CrossRef]
- Dai, J.; Tian, S.; Jiang, Y.; Chang, Z.; Xie, A.; Zhang, R.; Li, C.; Yan, Y. Fe3C/Fe/C magnetic hierarchical porous carbon with micromesopores for highly efficient chloramphenicol adsorption: Magnetization, graphitization, and adsorption properties investigation. Ind. Eng. Chem. Res. 2018, 57, 3510–3522. [Google Scholar] [CrossRef]
- Zuo, P.; Duan, J.; Fan, H.; Qu, S.; Shen, W. Facile synthesis high nitrogen-doped porous carbon nanosheet from pomelo peel and as catalyst support for nitrobenzene hydrogenation. Appl. Surf. Sci. 2018, 435, 1020–1028. [Google Scholar] [CrossRef]
- Li, Q.; Liu, F.; Zhang, L.; Nelson, B.J.; Zhang, S.; Ma, C.; Tao, X.; Cheng, J.; Zhang, X. In situ construction of potato starch based carbon nanofiber/activated carbon hybrid structure for high-performance electrical double layer capacitor. J. Power Sources 2012, 207, 199–204. [Google Scholar] [CrossRef]
- Tu, J.L.; Ding, M.Y.; Zhang, Q.; Zhang, Y.L.; Wang, C.G.; Wang, T.J.; Ma, L.L.; Li, X.J. Design of carbon-encapsulated Fe3O4 nanocatalyst with enhanced performance for Fischer–Tropsch synthesis. ChemCatChem 2015, 7, 2323–2327. [Google Scholar] [CrossRef]
- Song, F.; Yong, X.; Wu, X.; Zhang, W.; Ma, Q.; Zhao, T.; Tan, M.; Guo, Z.; Zhao, H.; Yang, G.; et al. FeMn@HZSM-5 capsule catalyst for light olefins direct synthesis via Fischer–Tropsch synthesis: Studies on depressing the CO2 formation. Appl. Catal. B Environ. 2022, 300, 120713–120722. [Google Scholar] [CrossRef]
- Guo, L.; Sun, J.; Ji, X.; Wei, J.; Wen, Z.; Yao, R.; Xu, H.; Ge, Q. Directly converting carbon dioxide to linear α-olefins on bio-promoted catalysts. Commun. Chem. 2018, 1, 51–59. [Google Scholar] [CrossRef]
- de Smit, E.; Weckhuysen, B.M. The renaissance of iron-based Fischer–Tropsch synthesis: On the multifaceted catalyst deactivation behaviour. Chem. Soc. Rev. 2008, 37, 2758–2781. [Google Scholar] [CrossRef]
- Xu, J.D.; Chang, Z.Y.; Zhu, K.T.; Weng, X.F.; Weng, W.Z.; Zheng, Y.P.; Huang, C.J.; Wan, H.L. Effect of sulfur on α-Al2O3-supported iron catalyst for Fischer–Tropsch synthesis. Appl. Catal. A Gen. 2016, 514, 103–113. [Google Scholar] [CrossRef]
- Amoyal, M.; Vidruk-Nehemya, R.; Landau, M.V.; Herskowitz, M. Effect of potassium on the active phases of Fe catalysts for carbon dioxide conversion to liquid fuels through hydrogenation. J. Catal. 2017, 348, 29–39. [Google Scholar] [CrossRef]
- Gong, J.; Cao, C.; Sun, R.; Cui, L.; Gao, R.; Hao, H. A DFT insight into the tuning effect of potassium promoter on the formation of carbon atoms via carburization gases dissociation on iron-based catalysts. Catalysts 2020, 10, 527. [Google Scholar] [CrossRef]
- Tian, Z.; Wang, C.; Yue, J.; Zhang, X.; Ma, L. Effect of a potassium promoter on the Fischer–Tropsch synthesis of light olefins over iron carbide catalysts encapsulated in graphene-like carbon. Catal. Sci. Technol. 2019, 9, 2728–2741. [Google Scholar] [CrossRef]
- Qiu, T.; Wang, L.; Lv, S.; Sun, B.; Zhang, Y.; Liu, Z.; Yang, W.; Li, J. SAPO-34 zeolite encapsulated Fe3C nanoparticles as highly selective Fischer–Tropsch catalysts for the production of light olefins. Fuel 2017, 203, 811–816. [Google Scholar] [CrossRef]
- Li, J.; Yu, F.; Wang, M.; Lai, Y.; Wang, H.; Lei, X.; Fang, J. Highly dispersed iron nitride nanoparticles embedded in N doped carbon as a high performance electrocatalyst for oxygen reduction reaction. Int. J. Hydrogen Energy 2017, 42, 2996–3005. [Google Scholar] [CrossRef]
- Huang, R.; Cao, M.; Guo, H.; Qi, W.; Su, R.; He, Z. Enhanced ethanol production from pomelo peel waste by integrated hydrothermal treatment, multienzyme formulation, and fed-batch operation. J. Agric. Food Chem. 2014, 62, 4643–4651. [Google Scholar] [CrossRef]
- Rivas, B.; Torrado, A.; Torre, P.; Converti, A.; Domínguez, J.M. Submerged citric acid fermentation on orange peel autohydrolysate. J. Agric. Food Chem. 2008, 56, 2380–2387. [Google Scholar] [CrossRef]
- Patsalou, M.; Samanides, C.G.; Protopapa, E.; Stavrinou, S.; Vyrides, I.; Koutinas, M. A Citrus peel waste biorefinery for ethanol and methane production. Molecules 2019, 24, 2451–2466. [Google Scholar] [CrossRef]
- Chai, W.; Liu, X.; Zou, J.; Zhang, X.; Li, B.; Yin, T. Pomelo peel modified with acetic anhydride and styrene as new sorbents for removal of oil pollution. Carbohydr. Polym. 2015, 132, 245–251. [Google Scholar] [CrossRef]
- Park, H.; Youn, D.H.; Kim, J.Y.; Kim, W.Y.; Choi, Y.H.; Lee, Y.H.; Choi, S.H.; Lee, J.S. Selective formation of Hägg iron carbide with g-C3N4 as a sacrificial support for highly active Fischer–Tropsch synthesis. ChemCatChem 2015, 7, 3488–3494. [Google Scholar] [CrossRef]
- Schulte, H.J.; Graf, B.; Xia, W.; Muhler, M. Nitrogen- and oxygen-functionalized multiwalled carbon nanotubes used as support in iron-catalyzed, high-temperature Fischer–Tropsch synthesis. ChemCatChem 2012, 4, 350–355. [Google Scholar] [CrossRef]
- Wu, J.; Wang, L.; Lv, B.; Chen, J. Facile fabrication of BCN nanosheet-encapsulated nano-iron as highly stable Fischer–Tropsch synthesis catalyst. ACS Appl. Mater. Interfaces 2017, 9, 14319–14327. [Google Scholar]
- Yan, Q.; Wan, C.; Liu, J.; Gao, J.; Yu, F.; Zhang, J.; Cai, Z. Iron nanoparticles in situ encapsulated in biochar-based carbon as an effective catalyst for the conversion of biomass-derived syngas to liquid hydrocarbons. Green Chem. 2013, 15, 1631–1641. [Google Scholar] [CrossRef]
- Jiang, F.; Liu, B.; Li, W.; Zhang, M.; Li, Z.; Liu, X. Two-dimensional graphene-directed formation of cylindrical iron carbide nanocapsules for Fischer–Tropsch synthesis. Catal. Sci. Technol. 2017, 7, 4609–4621. [Google Scholar] [CrossRef]
- Gu, B.; He, S.; Zhou, W.; Kang, J.; Cheng, K.; Zhang, Q.; Wang, Y. Polyaniline-supported iron catalyst for selective synthesis of lower olefins from syngas. J. Energy Chem. 2017, 26, 608–615. [Google Scholar] [CrossRef]
- Xie, J.; Galvis, H.M.T.; Koeken, A.C.J.; Kirilin, A.; Dugulan, A.I.; Ruitenbeek, M.; de Jong, K.P. Size and promoter effects on stability of carbon-nanofiber-supported iron-based Fischer–Tropsch catalysts. ACS Catal. 2016, 6, 4017–4024. [Google Scholar] [CrossRef]
- Bahome, M.C.; Jewell, L.L.; Padayachy, K.; Hildebrandt, D.; Glasser, D.; Datye, A.K.; Coville, N.J. Fe-Ru small particle bimetallic catalysts supported on carbon nanotubes for use in Fischer–Tropsch synthesis. Appl. Catal. A-Gen. 2007, 328, 243–251. [Google Scholar] [CrossRef]
- Tian, Z.; Wang, C.; Si, Z.; Ma, L.; Chen, L.; Liu, Q.; Zhang, Q.; Huang, H. Fischer–Tropsch synthesis to light olefins over iron-based catalysts supported on KMnO4 modified activated carbon by a facile method. Appl. Catal. A-Gen. 2017, 541, 50–59. [Google Scholar] [CrossRef]
- Zhao, X.; Lv, S.; Wang, L.; Li, L.; Wang, G.; Zhang, Y.; Li, J. Comparison of preparation methods of iron-based catalysts for enhancing Fischer–Tropsch synthesis performance. Mol. Catal. 2018, 449, 99–105. [Google Scholar] [CrossRef]









| Sample | Phase | IS (mm·s−1) | QS (mm·s−1) | H (kOe) | Spectral Contribution (%) |
|---|---|---|---|---|---|
| Fe@C-1.0 | θ-Fe3C | 0.19 | 0.02 | 210 | 75.2 |
| α-Fe | 0.01 | - | 330 | 18.0 | |
| Fe3+ (spm) | 0.20 | 1.01 | - | 6.9 |
| Samples | Fe a (wt%) | K a (wt%) | Surface Area b (m2·g−1) | Pore Volume b (cm3·g−1) | Pore Diameter b (nm) | ID/IG c |
|---|---|---|---|---|---|---|
| Pomelo peel e | <0.1 | 1.1 | 0.6 | <0.01 | 14.6 | - d |
| Carbonized pomelo peel f | <0.1 | 3.9 | 1.5 | <0.01 | 11.2 | 3.13 |
| Fe@C-3.0 | 13.5 | 3.9 | 91.0 | 0.14 | 4.0 | 2.36 |
| Fe@C-1.5 | 23.9 | 3.9 | 90.6 | 0.21 | 8.5 | 1.96 |
| Fe@C-1.0 | 36.7 | 3.2 | 64.6 | 0.23 | 6.7 | 1.72 |
| Fe@C-0.67 | 47.2 | 2.2 | 48.1 | 0.18 | 8.7 | 1.67 |
| Catalysts | Fe a (wt%) | CO Conversion (%) | Selectivity of CO2 (C%) | Hydrocarbon Selectivity (wt%, Free of CO2) | C2-4 O/P b | α-Chain Growth Probability Factor c | |||
|---|---|---|---|---|---|---|---|---|---|
| CH4 | C2-40 | C2-4= | C5+ | ||||||
| Fe@C-6.0 | 8.0 | 19.5 | 63.3 | 22.5 | 8.0 | 45.7 | 23.8 | 5.7 | - d |
| Fe@C-4.0 | 10.8 | 40.7 | 61.1 | 16.1 | 6.2 | 40.5 | 37.2 | 6.5 | 0.839 |
| Fe@C-3.0 | 13.5 | 65.0 | 56.8 | 12.2 | 5.9 | 32.5 | 49.4 | 5.5 | 0.825 |
| Fe@C-2.0 | 18.8 | 87.2 | 56.1 | 10.7 | 5.8 | 29.7 | 53.8 | 5.1 | 0.819 |
| Fe@C-1.5 | 23.9 | 90.8 | 54.0 | 9.1 | 4.6 | 27.4 | 58.9 | 5.9 | 0.813 |
| Fe@C-1.0 | 36.7 | 92.6 | 52.3 | 8.5 | 4.2 | 26.0 | 61.3 | 6.2 | 0.833 |
| Fe@C-0.86 | 42.4 | 91.8 | 53.4 | 8.6 | 4.4 | 26.4 | 60.6 | 6.1 | 0.831 |
| Fe@C-0.67 | 47.2 | 79.2 | 55.5 | 7.5 | 4.1 | 30.7 | 57.8 | 7.6 | 0.790 |
| Catalysts | Fe a (wt%) | K a (wt%) | CO Conversion (%) | Selectivity of CO2 (C%) | Hydrocarbon Selectivity (wt%, Free of CO2) | C2-4 O/P | α-Chain Growth Probability Factor | |||
|---|---|---|---|---|---|---|---|---|---|---|
| CH4 | C2-40 | C2-4= | C5+ | |||||||
| Fe@C-1.0 | 36.7 | 3.2 | 92.6 | 52.3 | 8.5 | 4.2 | 26.0 | 61.3 | 6.2 | 0.833 |
| Fe@C-1.0-W b | 35.1 | 0.1 | 94.3 | 51.6 | 23.0 | 30.1 | 8.3 | 38.5 | 0.3 | 0.794 |
| Fe@C-1.0-W-IM c | 31.7 | 2.7 | 83.4 | 57.8 | 8.7 | 4.9 | 31.0 | 55.4 | 6.3 | 0.802 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, S.; Chen, J.; Fan, Y.; Huang, Z.; Meng, Q.; Ma, L.; Zhang, Q.; Wang, T. Direct Construction of K-Fe3C@C Nanohybrids Utilizing Waste Biomass of Pomelo Peel as High-Performance Fischer–Tropsch Catalysts. Catalysts 2022, 12, 542. https://doi.org/10.3390/catal12050542
Qiu S, Chen J, Fan Y, Huang Z, Meng Q, Ma L, Zhang Q, Wang T. Direct Construction of K-Fe3C@C Nanohybrids Utilizing Waste Biomass of Pomelo Peel as High-Performance Fischer–Tropsch Catalysts. Catalysts. 2022; 12(5):542. https://doi.org/10.3390/catal12050542
Chicago/Turabian StyleQiu, Songbai, Jianfeng Chen, Yujian Fan, Zan Huang, Qingwei Meng, Liang Ma, Qian Zhang, and Tiejun Wang. 2022. "Direct Construction of K-Fe3C@C Nanohybrids Utilizing Waste Biomass of Pomelo Peel as High-Performance Fischer–Tropsch Catalysts" Catalysts 12, no. 5: 542. https://doi.org/10.3390/catal12050542