Olive Mill Wastewater (OMW) Treatment Using Photocatalyst Media
Abstract
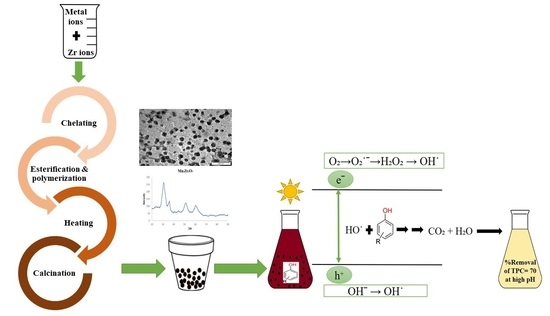
:1. Introduction
2. Results
2.1. The Chemical Structure of the Prepared Nano Photocatalysts
2.2. Characterization of the Prepared Nano Photocatalysts
2.3. Photocatalytic Activity of the Prepared Material on OMW Treatment
3. Materials and Methods
3.1. Materials
3.2. Preparation of Photocatalysts
3.3. Characterization of the Prepared Material
3.4. OMW Pretreatment
3.5. Evaluation of Photocatalytic Activity
3.6. Spectrometric Measurement of Total Phenolic Compounds (TPCs)
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hussain, M.I.; Muscolo, A.; Farooq, M.; Ahmad, W. Sustainable Use and Management of Non-Conventional Water Resources for Rehabilitation of Marginal Lands in Arid and Semiarid Environments. Agric. Water Manag. 2019, 221, 462–476. [Google Scholar] [CrossRef]
- Saidan, M.N.; Al-Addous, M.; Al-Weshah, R.A.; Obada, I.; Alkasrawi, M.; Barbana, N. Wastewater Reclamation in Major Jordanian Industries: A Viable Component of a Circular Economy. Water 2020, 12, 1276. [Google Scholar] [CrossRef]
- Tsagaraki, E.; Lazarides, H.N.; Petrotos, K.B. Olive Mill Wastewater Treatment. In Utilization of By-Products and Treatment of Waste in the Food Industry; Oreopoulou, V., Russ, W., Eds.; Springer US: Boston, MA, USA, 2007; pp. 133–157. [Google Scholar]
- Hytiris, N.; Kapellakis, I.E.; Tsagarakis, K.P. The Potential Use of Olive Mill Sludge in Solidification Process. Resour. Conserv. Recycl. 2004, 40, 129–139. [Google Scholar] [CrossRef]
- Ayoub, S.; Al-Absi, K.; Al-Shdiefat, S.; Al-Majali, D.; Hijazean, D. Effect of Olive Mill Wastewater Land-Spreading on Soil Properties, Olive Tree Performance and Oil Quality. Sci. Hortic. 2014, 175, 160–166. [Google Scholar] [CrossRef]
- Federici, F.; Fava, F.; Kalogerakis, N.; Mantzavinos, D. Valorisation of Agro-industrial By-products, Effluents and Waste: Concept, Opportunities and the Case of Olive Mill Wastewaters. J. Chem. Technol. Biotechnol. Int. Res. Process Environ. Clean Technol. 2009, 84, 895–900. [Google Scholar] [CrossRef]
- Papaoikonomou, L.; Labanaris, K.; Kaderides, K.; Goula, A.M. Adsorption–Desorption of Phenolic Compounds from Olive Mill Wastewater Using a Novel Low-Cost Biosorbent. Environ. Sci. Pollut. Res. 2021, 28, 24230–24244. [Google Scholar] [CrossRef]
- González-González, A.; Cuadros, F. Effect of Aerobic Pretreatment on Anaerobic Digestion of Olive Mill Wastewater (OMWW): An Ecoefficient Treatment. Food Bioprod. Processing 2015, 95, 339–345. [Google Scholar] [CrossRef]
- Cassano, A.; Conidi, C.; Giorno, L.; Drioli, E. Fractionation of Olive Mill Wastewaters by Membrane Separation Techniques. J. Hazard. Mater. 2013, 248–249, 185–193. [Google Scholar] [CrossRef]
- Esteves, B.M.; Rodrigues, C.S.D.; Maldonado-Hódar, F.J.; Madeira, L.M. Treatment of High-Strength Olive Mill Wastewater by Combined Fenton-like Oxidation and Coagulation/Flocculation. J. Environ. Chem. Eng. 2019, 7, 103252. [Google Scholar] [CrossRef]
- Stoller, M.; Azizova, G.; Mammadova, A.; Vilardi, G.; Di Palma, L.; Chianese, A. Treatment of Olive Oil Processing Wastewater by Ultrafiltration, Nanofiltration, Reverse Osmosis and Biofiltration. Chem. Eng. Trans. 2016, 47, 409–414. [Google Scholar] [CrossRef]
- Elkacmi, R.; Bennajah, M. Advanced Oxidation Technologies for the Treatment and Detoxification of Olive Mill Wastewater: A General Review. J. Water Reuse Desalination 2019, 9, 463–505. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Pignatello, J.J.; Ma, J.; Mitch, W.A. Effect of Matrix Components on UV/H2O2 and UV/S2O82− Advanced Oxidation Processes for Trace Organic Degradation in Reverse Osmosis Brines from Municipal Wastewater Reuse Facilities. Water Res. 2016, 89, 192–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esteves, B.M.; Rodrigues, C.S.D.; Madeira, L.M. Synthetic Olive Mill Wastewater Treatment by Fenton’s Process in Batch and Continuous Reactors Operation. Environ. Sci. Pollut. Res. 2018, 25, 34826–34838. [Google Scholar] [CrossRef] [PubMed]
- Kallel, M.; Belaid, C.; Boussahel, R.; Ksibi, M.; Montiel, A.; Elleuch, B. Olive Mill Wastewater Degradation by Fenton Oxidation with Zero-Valent Iron and Hydrogen Peroxide. J. Hazard. Mater. 2009, 163, 550–554. [Google Scholar] [CrossRef]
- Benitez, F.J.; Beltran-Heredia, J.; Torregrosa, J.; Acero, J.L. Treatment of Olive Mill Wastewaters by Ozonation, Aerobic Degradation and the Combination of Both Treatments. J. Chem. Technol. Biotechnol. 1999, 74, 639–646. [Google Scholar] [CrossRef]
- Hodaifa, G.; Ochando-Pulido, J.M.; Rodriguez-Vives, S.; Martinez-Ferez, A. Optimization of Continuous Reactor at Pilot Scale for Olive-Oil Mill Wastewater Treatment by Fenton-like Process. Chem. Eng. J. 2013, 220, 117–124. [Google Scholar] [CrossRef]
- Benitez, F.J.; Beltran-Heredia, J.; Torregrosa, J.; Acero, J.L. Treatments of Wastewaters from Olive Oil Mills by Uv Radiation and by Combined Ozone-UV Radiation. Toxicol. Environ. Chem. 1997, 61, 173–185. [Google Scholar] [CrossRef]
- García, C.A.; Hodaifa, G. Real Olive Oil Mill Wastewater Treatment by Photo-Fenton System Using Artificial Ultraviolet Light Lamps. J. Clean. Prod. 2017, 162, 743–753. [Google Scholar] [CrossRef]
- El Hajjouji, H.; Barje, F.; Pinelli, E.; Bailly, J.-R.; Richard, C.; Winterton, P.; Revel, J.-C.; Hafidi, M. Photochemical UV/TiO2 Treatment of Olive Mill Wastewater (OMW). Bioresour. Technol. 2008, 99, 7264–7269. [Google Scholar] [CrossRef] [Green Version]
- Atanassova, D.; Kefalas, P.; Petrakis, C.; Mantzavinos, D.; Kalogerakis, N.; Psillakis, E. Sonochemical Reduction of the Antioxidant Activity of Olive Mill Wastewater. Environ. Int. 2005, 31, 281–287. [Google Scholar] [CrossRef]
- Jum’h, I.; Abdelhay, A.; Al-Taani, H.; Telfah, A.; Alnaief, M.; Rosiwal, S. Fabrication and Application of Boron Doped Diamond BDD Electrode in Olive Mill Wastewater Treatment in Jordan. J. Water Reuse Desalination 2016, 7, 502–510. [Google Scholar] [CrossRef]
- Bellakhal, N.; Oturan, M.; Oturan, N.; Dachraoui, M. Olive Oil Mill Wastewater Treatment by the Electro-Fenton Process. Environ. Chem. 2006, 3, 345–349. [Google Scholar] [CrossRef]
- Amor, C.; Lucas, M.S.; García, J.; Dominguez, J.R.; De Heredia, J.B.; Peres, J.A. Combined Treatment of Olive Mill Wastewater by Fenton’s Reagent and Anaerobic Biological Process. J. Environ. Sci. Health 2015, 50, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Madani, M.; Aliabadi, M.; Nasernejad, B.; Kermanj Abdulrahman, R.; Yalili Kilic, M.; Kestioglu, K. Treatment of Olive Mill Wastewater Using Physico-Chemical and Fenton Processes. Desalination Water Treat. 2015, 53, 2031–2040. [Google Scholar] [CrossRef]
- Gernjak, W.; Maldonado, M.I.; Malato, S.; Cáceres, J.; Krutzler, T.; Glaser, A.; Bauer, R. Pilot-Plant Treatment of Olive Mill Wastewater (OMW) by Solar TiO2 Photocatalysis and Solar Photo-Fenton. Sol. Energy 2004, 77, 567–572. [Google Scholar] [CrossRef]
- Al-Bsoul, A.; Al-Shannag, M.; Tawalbeh, M.; Al-Taani, A.A.; Lafi, W.K.; Al-Othman, A.; Alsheyab, M. Optimal Conditions for Olive Mill Wastewater Treatment Using Ultrasound and Advanced Oxidation Processes. Sci. Total Environ. 2020, 700, 134576. [Google Scholar] [CrossRef]
- Iboukhoulef, H.; Douani, R.; Amrane, A.; Chaouchi, A.; Elias, A. Heterogeneous Fenton like Degradation of Olive Mill Wastewater Using Ozone in the Presence of BiFeO3 Photocatalyst. J. Photochem. Photobiol. A Chem. 2019, 383, 112012. [Google Scholar] [CrossRef]
- Lim, T.-T.; Yap, P.-S.; Srinivasan, M.; Fane, A.G. TiO2/AC Composites for Synergistic Adsorption-Photocatalysis Processes: Present Challenges and Further Developments for Water Treatment and Reclamation. Crit. Rev. Environ. Sci. Technol. 2011, 41, 1173–1230. [Google Scholar] [CrossRef]
- Yahya, N.; Aziz, F.; Jamaludin, N.A.; Mutalib, M.A.; Ismail, A.F.; Salleh, W.N.; Jaafar, J.; Yusof, N.; Ludin, N.A. A Review of Integrated Photocatalyst Adsorbents for Wastewater Treatment. J. Environ. Chem. Eng. 2018, 6, 7411–7425. [Google Scholar] [CrossRef]
- Bahnemann, D.; Bockelmann, D.; Goslich, R. Mechanistic Studies of Water Detoxification in Illuminated TiO2 Suspensions. Sol. Energy Mater. 1991, 24, 564–583. [Google Scholar] [CrossRef]
- Thiruvenkatachari, R.; Vigneswaran, S.; Moon, I.S. A Review on UV/TiO2 Photocatalytic Oxidation Process (Journal Review). Korean J. Chem. Eng. 2008, 25, 64–72. [Google Scholar] [CrossRef]
- Zangeneh, H.; Zinatizadeh, A.A.L.; Habibi, M.; Akia, M.; Hasnain Isa, M. Photocatalytic Oxidation of Organic Dyes and Pollutants in Wastewater Using Different Modified Titanium Dioxides: A Comparative Review. J. Ind. Eng. Chem. 2015, 26, 1–36. [Google Scholar] [CrossRef]
- Al-Mamun, M.R.; Kader, S.; Islam, M.S.; Khan, M.Z.H. Photocatalytic Activity Improvement and Application of UV-TiO2 Photocatalysis in Textile Wastewater Treatment: A Review. J. Environ. Chem. Eng. 2019, 7, 103248. [Google Scholar] [CrossRef]
- Xu, P.; Zeng, G.M.; Huang, D.L.; Feng, C.L.; Hu, S.; Zhao, M.H.; Lai, C.; Wei, Z.; Huang, C.; Xie, G.X.; et al. Use of Iron Oxide Nanomaterials in Wastewater Treatment: A Review. Sci. Total Environ. 2012, 424, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.M.; Lai, C.W.; Ngai, K.S.; Juan, J.C. Recent Developments of Zinc Oxide Based Photocatalyst in Water Treatment Technology: A Review. Water Res. 2016, 88, 428–448. [Google Scholar] [CrossRef]
- Ruzmanova, I.; Ustundas, M.; Stoller, M.; Chianese, A. Photocatalytic Treatment of Olive Mill Wastewater by N-Doped Titanium Dioxide Nanoparticles under Visible Light. Chem. Eng. Trans. 2013, 32, 2233–2238. [Google Scholar] [CrossRef]
- Nogueira, V.; Lopes, I.; Rocha-Santos, T.A.P.; Gonçalves, F.; Duarte, A.C.; Pereira, R. Photocatalytic Treatment of Olive Oil Mill Wastewater Using TiO2 and Fe2O3 Nanomaterials. Water Air Soil Pollut. 2016, 227, 88. [Google Scholar] [CrossRef]
- Sponza, D.T.; Oztekin, R. Treatment of Olive Mill Wastewater by Photooxidation with ZrO2-Doped TiO2 Nanocomposite and Its Reuse Capability. Environ. Technol. 2016, 37, 865–879. [Google Scholar] [CrossRef]
- Alicanoğlu, P.; Ulusoy, Ç.; Sponza, D. Effect of Graphene-TiO2 on the Photodegradation of Olive Mill Effluent and Recovery of Graphene-TiO2. Sigma J. Eng. Nat. Sci. 2017, 8, 227–234. [Google Scholar]
- Al Bawab, A.; Ghannam, N.; Abu-Mallouh, S.; Bozeya, A.; Abu-Zurayk, R.A.; Al-Ajlouni, Y.A.; Odeh, F.; Abu-Dalo, M.A. Olive Mill Wastewater Treatment in Jordan: A Review; IOP Publishing: Irbid, Jordan, 2018; ICAM-2017; Volume 305, p. 012002. [Google Scholar]
- Odeh, F.; Bawab, A.; Fayyad, M.; Bozeya, A. Surfactant Enhanced Olive Oil Mill Wastewater Remediation. APCBEE Procedia 2013, 5, 96–101. [Google Scholar] [CrossRef] [Green Version]
- Al-Bawab, A.; Alshawawreh, F.; Abu-Dalo, M.A.; Al-Rawashdeh, N.A.; Bozeya, A. Separation of Soluble Phenolic Compounds from Olive Mill Wastewater (OMW) Using Modified Surfactant. Fresenius Environ. Bull. 2017, 26, 1949–1958. [Google Scholar]
- Al-Bawab, A.; Ghannam, N.; Abu-Zurayk, R.A.; Odeh, F.; Bozeya, A.; Mallouh, S.; Al-Rawashdeh, N.A.; Abu-Dalo, M.A. Olive Mill Wastewater Remediation by Granular Activated Carbon Impregnated with Active Materials. Fresenius Environ. Bull. 2018, 27, 2118–2126. [Google Scholar]
- Abu-Dalo, M.; Abdelnabi, J.; Al-Rawashdeh, N.A.; Albiss, B.; Al Bawab, A. Coupling Coagulation-Flocculation to Volcanic Tuff-Magnetite Nanoparticles Adsorption for Olive Mill Wastewater Treatment. Environ. Nanotechnol. Monit. Manag. 2022, 17, 100626. [Google Scholar] [CrossRef]
- Abu-Dalo, M.; Abdelnabi, J.; Bawab, A.A. Preparation of Activated Carbon Derived from Jordanian Olive Cake and Functionalized with Cu/Cu2O/CuO for Adsorption of Phenolic Compounds from Olive Mill Wastewater. Materials 2021, 14, 6636. [Google Scholar] [CrossRef]
- Kanaan, K. Coupling Activated Carbon with Surface Active Materials for Olive Mill Wastewater Treatment; Jordan University of Science and Technology: Irbid, Jordan, 2017. [Google Scholar]
- Abu-Dalo, M.A.; Al-Atoom, M.A.; Aljarrah, M.T.; Albiss, B.A. Preparation and Characterization of Polymer Membranes Impregnated with Carbon Nanotubes for Olive Mill Wastewater. Polymers 2022, 14, 457. [Google Scholar] [CrossRef]
- Abbas, H.A.; Nasr, R.A.; Khalaf, A.; Al Bawab, A.; Jamil, T.S. Photocatalytic Degradation of Methylene Blue Dye by Fluorite Type Fe2Zr2-xWxO7 System under Visible Light Irradiation. Ecotoxicol. Environ. Saf. 2020, 196, 110518. [Google Scholar] [CrossRef]
- Abbas, H.; Nasr, R.A.; Abu-Zurayk, R.; Al Bawab, A.; Jamil, T.S. Decolourization of Crystal Violet Using Nano-Sized Novel Fluorite Structure Ga2Zr2−xWxO7 Photocatalyst under Visible Light Irradiation. R. Soc. Open Sci. 2020, 7, 191632. [Google Scholar] [CrossRef] [Green Version]
- Nasr, R.A.; Abbas, H.; Khalaf, A.; Bozeya, A.; Jamil, T.S. Nano-Sized Ga2-xCuxZr2-xWxO7 for Malachite Green Decolorization under Visible Light. Desalination Water Treat. 2020, 183, 389–403. [Google Scholar] [CrossRef]
- Abu-Zurayk, R.; Khalaf, A.; Abbas, H.A.; Nasr, R.A.; Jamil, T.S.; Al Bawab, A. Photodegradation of Carbol Fuchsin Dye Using an Fe2−xCuxZr2−xWxO7 Photocatalyst under Visible-Light Irradiation. Catalysts 2021, 11, 1473. [Google Scholar] [CrossRef]
- Thampi, V.; Padala, P.R.; Radhakrishnan, A.N. Induced Oxygen Vacancies and Their Effect on the Structural and Electrical Properties of a Fluorite-Type CaZrO3–Gd2Zr2O7 System. New J. Chem. 2015, 39, 1469–1476. [Google Scholar] [CrossRef]
- Suresh, S.; Karthikeyan, S.; Jayamoorthy, K. FTIR and Multivariate Analysis to Study the Effect of Bulk and Nano Copper Oxide on Peanut Plant Leaves. J. Sci. Adv. Mater. Devices 2016, 1, 343–350. [Google Scholar] [CrossRef] [Green Version]
- Chary, K.; Guggilla, V.; Chakravartula Srivatsa, S.; Rao, V. Characterization and Catalytic Functionalities of Copper Oxide Catalysts Supported on Zirconia. J. Phys. Chem. B 2007, 111, 543–550. [Google Scholar] [CrossRef] [PubMed]
- Vallet-Regí, M.; Romero, A.M.; Ragel, C.V.; LeGeros, R.Z. XRD, SEM-EDS, and FTIR Studies of in Vitro Growth of an Apatite-like Layer on Sol-Gel Glasses. J. Biomed. Mater. Res. 1999, 44, 416–421. [Google Scholar] [CrossRef]
- He, H.-Y. Photocatalytic Degradations of Dyes on Magnetically Separable Ni1−xCoxFe2O4 Nanoparticles Synthesized by a Hydrothermal Process. Part. Sci. Technol. 2015, 34, 150713172707003. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised Effective Ionic Radii and Systematic Studies of Interatomic Distances in Halides and Chalcogenides. Acta Crystallogr. Sect. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Benreguia, N.; Barnabé, A.; Trari, M. Sol–Gel Synthesis and Characterization of the Delafossite CuAlO2. J. Sol-Gel Sci. Technol. 2015, 75, 670–679. [Google Scholar] [CrossRef] [Green Version]
- Reddy, V.; Bandaru, N.; Shim, J.; Vattikuti, S. Synthesis of CdO/ZnS Heterojunction for Photodegradation of Organic Dye Molecules. Appl. Phys. A 2017, 123, 1–12. [Google Scholar]
- Zuccaro, L.; Krieg, J.; Desideri, A.; Kern, K.; Balasubramanian, K. Tuning the Isoelectric Point of Graphene by Electrochemical Functionalization. Sci. Rep. 2015, 5, 11794. [Google Scholar] [CrossRef] [Green Version]
- Nogueira, V.; Lopes, I.; Freitas, A.C.; Rocha-Santos, T.A.P.; Gonçalves, F.; Duarte, A.C.; Pereira, R. Biological Treatment with Fungi of Olive Mill Wastewater Pre-Treated by Photocatalytic Oxidation with Nanomaterials. Ecotoxicol. Environ. Saf. 2015, 115, 234–242. [Google Scholar] [CrossRef]
- Baransi, K.; Dubowski, Y.; Sabbah, I. Synergetic Effect between Photocatalytic Degradation and Adsorption Processes on the Removal of Phenolic Compounds from Olive Mill Wastewater. Water Res. 2012, 46, 789–798. [Google Scholar] [CrossRef] [PubMed]
- Uğurlu, M.; Karaoğlu, M.H. TiO2 Supported on Sepiolite: Preparation, Structural and Thermal Characterization and Catalytic Behaviour in Photocatalytic Treatment of Phenol and Lignin from Olive Mill Wastewater. Chem. Eng. J. 2011, 166, 859–867. [Google Scholar] [CrossRef]
- Badawy, M.I.; Gohary, F.E.; Ghaly, M.Y.; Ali, M.E.M. Enhancement of Olive Mill Wastewater Biodegradation by Homogeneous and Heterogeneous Photocatalytic Oxidation. J. Hazard. Mater. 2009, 169, 673–679. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.H.; Chu, W. The Dose and Ratio Effects of Fe(II) and H2O2 in Fenton’s Process on the Removal of Atrazine. Environ. Technol. 2003, 24, 703–710. [Google Scholar] [CrossRef]
- Pechini, M.P. Method of Preparing Lead and Alkaline Earth Titanates and Niobates and Coating Method Using the Same to Form a Capacitor. U.S. Patent 3,330,697, 11 July 1967. [Google Scholar]
- Leouifoudi, I.; Harnafi, H.; Zyad, A. Olive Mill Waste Extracts: Polyphenols Content, Antioxidant, and Antimicrobial Activities. Adv. Pharmacol. Sci. 2015, 2015, 714138. [Google Scholar] [CrossRef]












| Photocatalyst System | Photocatalyst Structure | Abbreviation |
|---|---|---|
| M2Zr2O7 | Fe2Zr2O7 | FeZr |
| Cu2Zr2O7 | CuZr | |
| Mn2Zr2O7 | MnZr | |
| Fe2−xMnxZr2O7 | Fe1.85Mn0.15Zr2O7 | FeMn1 |
| Fe1.70Mn0.30Zr2O7 | FeMn2 | |
| Fe2−xCuxZr2O7 | Fe1.85Cu0.15Zr2O7 | FeCu1 |
| Fe1.70Cu0.30Zr2O7 | FeCu2 | |
| Fe2Zr2−xWxO7 | Fe2Zr1.85W0.15O7 | ZrW1 |
| Fe2Zr1.70W0.30O7 | ZrW2 |
| Photocatalyst | Average Particle Size (nm) |
|---|---|
| FeZr | 6.40 |
| CuZr | 17.0 |
| MnZr | 7.80 |
| FeMn1 | 6.10 |
| FeMn2 | 6.80 |
| FeCu1 | 6.90 |
| FeCu2 | 7.40 |
| ZrW1 | 5.90 |
| ZrW2 | 6.00 |
| Parent Photocatalyst | Theoretical Formula | Theoretical Mole Ratio (A/B) | ICP-OES Results (ppm) | mol/L | Experimental Mole Ratio |
|---|---|---|---|---|---|
| FeZr | Fe2Zr2O7 | 1 | Fe = 1963.69 | 0.03516 | 0.99 |
| Zr = 3189.10 | 0.03496 | ||||
| CuZr | Cu2Zr2O7 | 1 | Cu = 6521.50 | 0.10260 | 1.04 |
| Zr = 9005.32 | 0.09872 | ||||
| MnZr | Mn2Zr2O7 | 1 | Mn = 1150.90 | 0.02095 | 1.01 |
| Zr = 1911.03 | 0.02083 |
| Photocatalyst Structure | Surface Area (m2/g) | Total Pore Volume (cc/g) | Average Pore Diameter (A°) |
|---|---|---|---|
| MnZr | 30 | 0.0220 | 29.182 |
| CuZr | 62 | 0.0661 | 42.992 |
| FeZr | 67 | 0.1204 | 71.672 |
| FeMn1 | 173 | 0.1615 | 37.376 |
| FeMn2 | 187 | 0.1585 | 33.988 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al Bawab, A.; Abu-Dalo, M.; Khalaf, A.; Abu-Dalo, D. Olive Mill Wastewater (OMW) Treatment Using Photocatalyst Media. Catalysts 2022, 12, 539. https://doi.org/10.3390/catal12050539
Al Bawab A, Abu-Dalo M, Khalaf A, Abu-Dalo D. Olive Mill Wastewater (OMW) Treatment Using Photocatalyst Media. Catalysts. 2022; 12(5):539. https://doi.org/10.3390/catal12050539
Chicago/Turabian StyleAl Bawab, Abeer, Muna Abu-Dalo, Aya Khalaf, and Duaa Abu-Dalo. 2022. "Olive Mill Wastewater (OMW) Treatment Using Photocatalyst Media" Catalysts 12, no. 5: 539. https://doi.org/10.3390/catal12050539