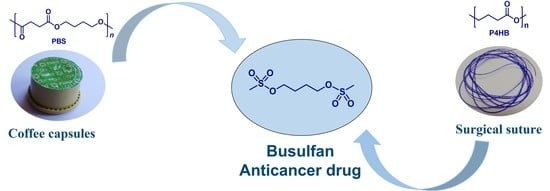
Depolymerization of P4HB and PBS Waste and Synthesis of the Anticancer Drug Busulfan from Plastic Waste
Abstract
:1. Introduction
2. Discussion and Results
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ellis, L.D.; Rorrer, N.A.; Sullivan, K.P.; Otto, M.; McGeehan, J.E.; Román-Leshkov, Y.; Wierckx, N.; Beskham, G.T. Chemical and Biological Catalysis for Plastics Deconstruction, Recycling, and Upcycling. Nat. Catal. 2021, 4, 539–556. [Google Scholar] [CrossRef]
- Estahbanati, M.R.K.; Kong, X.Y.; Eslami, A.; Soo, H.S. Current Developments in the Chemical Upcycling of Waste Plastics Using Alternative Energy Sources. ChemSusChem 2021, 14, 4152–4166. [Google Scholar] [CrossRef]
- Beghetto, V.; Sole, R.; Buranello, C.; Al-Abkal, M.; Facchin, M. Recent Advancements in Plastic Packaging Recycling: A Mini-Review. Materials 2021, 14, 4782. [Google Scholar] [CrossRef]
- Wang, C.; El-Sepelgy, O. Reductive depolymerization of plastics catalyzed with transition metal complexes. Curr. Opin. Green Sustain. Chem. 2021, 32, 100547. [Google Scholar] [CrossRef]
- Hou, Q.; Zhen, M.; Qian, H.; Nie, Y.; Bai, X.; Xia, T.; Rehman, M.L.U.; Li, Q.; Ju, M. Upcycling and catalytic degradation of plastic wastes. Cell Rep. Phys. Sci. 2021, 2, 100514. [Google Scholar] [CrossRef]
- Kumar, S.; Singh, E.; Mishra, R.; Kumar, A.; Caucci, S. Utilization of Plastic Wastes for Sustainable Environmental Management: A Review. ChemSusChem 2021, 14, 3985–4006. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Wan, K.; Zhang, Y.; Wang, Y. Waste to Wealth: Chemical Recycling and Chemical Upcycling of Waste Plastics for a Great Future. ChemSusChem 2021, 14, 4123–4136. [Google Scholar] [CrossRef] [PubMed]
- Qin, Z.-H.; Mou, J.-H.; Chao, C.Y.H.; Chopra, S.S.; Daoud, W.; Leu, S.-Y.; Ning, Z.; Tso, C.Y.; Chan, C.K.; Tang, S.; et al. Biotechnology of Plastic Waste Degradation, Recycling, and Valorization: Current Advances and Future Perspectives. ChemSusChem 2021, 14, 4103–4114. [Google Scholar] [CrossRef] [PubMed]
- Jönsson, C.; Wei, R.; Biundo, A.; Landberg, J.; Bour, L.S.; Pezzotti, F.; Toca, A.; Jacques, L.M.; Bornscheuer, U.T.; Syrén, P.-O. Biocatalysis in the Recycling Landscape for Synthetic Polymers and Plastics towards Circular Textiles. ChemSusChem 2021, 14, 4028–4040. [Google Scholar] [CrossRef] [PubMed]
- Miao, Y.; Jouanne, A.v.; Yokochi, A. Current Technologies in Depolymerization Process and the Road Ahead. Polymers 2021, 13, 449. [Google Scholar] [CrossRef]
- McKeown, P.; Jones, M.D. The Chemical Recycling of PLA: A Review. Sus. Chem. 2020, 1, 1–22. [Google Scholar] [CrossRef]
- Jiang, J.; Shi, K.; Zhang, X.; Yu, K.; Zhang, H.; He, J.; Ju, Y.; Liu, J. From plastic waste to wealth using chemical recycling: A Review. J. Environ. Chem. Eng. 2020, 10, 106867. [Google Scholar] [CrossRef]
- Sardon, H.; Dove, A.P. Organocatalysis for depolymerization. Polym. Chem. 2019, 10, 172–186. [Google Scholar]
- Tang, X.; Chen, E.Y.-X. Toward Infinitely Recyclable Plastics Derived from Renewable Cyclic Esters. Chem 2019, 5, 284–312. [Google Scholar] [CrossRef] [Green Version]
- Hong, M.; Chen, E.Y.-X. Chemically Recyclable Polymers: A Circular Economy Approach to Sustainability. Green Chem. 2017, 19, 3692–3706. [Google Scholar] [CrossRef]
- Fernandes, A.C. Reductive Depolymerization as an Efficient Methodology for the Conversion of Plastic Waste into Value-added Compounds. Green Chem. 2021, 23, 7330–7360. [Google Scholar] [CrossRef]
- Krall, E.M.; Klein, T.W.; Andersen, R.J.; Nett, A.J.; Glasgow, R.W.; Reader, D.S.; Dauphinais, B.C.; Mc Ilrath, S.P.; Fischer, A.A.; Carney, M.J.; et al. Controlled Hydrogenative Depolymerization of Polyesters and Polycarbonates Catalyzed by Ruthenium(II) PNN Pincer Complexes. Chem. Commun. 2014, 50, 4884–4887. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, J.A.; Smith, S.M.; Scharbert, M.T.; Carpenter, I.; Cordes, D.B.; Slawin, A.M.Z.; Clarke, M.L. On the Functional Group Tolerance of Ester Hydrogenation and Polyester Depolymerisation Catalysed by Ruthenium Complexes of Tridentate Aminophosphine Ligands. Chem. Eur. J. 2015, 21, 10851–10860. [Google Scholar] [CrossRef] [Green Version]
- Westhues, S.; Idel, J.; Klankermayer, J. Molecular catalyst systems as key enablers for tailored polyesters and polycarbonate recycling concepts. Sci. Adv. 2018, 4, eaat9669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feghali, E.; Cantat, T. Room Temperature Organocatalyzed Reductive Depolymerization of Waste Polyethers, Polyesters, and Polycarbonates. ChemSusChem 2015, 8, 980–984. [Google Scholar] [CrossRef] [PubMed]
- Monsigny, L.; Berthet, J.-C.; Cantat, T. Depolymerization of Waste Plastics to Monomers and Chemicals Using a Hydrosilylation Strategy Facilitated by Brookhart’s Iridium(III) Catalyst. ACS Sustain. Chem. Eng. 2018, 6, 10481–10488. [Google Scholar] [CrossRef]
- Shao, Z.; Zhong, R.; Ferraccioli, R.; Li, Y.; Liu, Q. General and Phosphine-Free Cobalt-Catalyzed Hydrogenation of Esters to Alcohols. Chin. J. Chem. 2019, 37, 1125–1130. [Google Scholar] [CrossRef]
- Farrar-Tobar, R.A.; Wozniak, B.; Savini, A.; Hinze, S.; Tin, S.; de Vries, J.G. Base-Free Iron Catalyzed Transfer Hydrogenation of Esters Using EtOH as Hydrogen Source. Angew. Chem. Int. Ed. 2019, 58, 1129–1133. [Google Scholar] [CrossRef] [PubMed]
- Nunes, B.F.S.; Oliveira, M.C.; Fernandes, A.C. Dioxomolybdenum Complex as an Efficient and Cheap Catalyst for the Reductive Depolymerization of Plastic Waste into Value-added Compounds and Fuels. Green Chem. 2020, 22, 2419–2425. [Google Scholar] [CrossRef]
- Fernandes, A.C. Reductive Depolymerization of Plastic Waste Catalyzed by Zn(OAc)2⋅2H2O. ChemSusChem 2021, 14, 4228–4233. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, A.C. Recent Advances in the Synthesis of Nitrogen Compounds from Biomass Derivatives. In Methodologies in Amine Synthesis. Challenges and Applications; Wiley-VCH: Weinheim, Germany, 2020; pp. 341–376. [Google Scholar]
- Elangovan, S.; Afanasenko, A.; Haupenthal, J.; Sun, Z.; Liu, Y.; Hirsch, A.K.H.; Barta, K. From Wood to Tetrahydro-2-benzazepines in Three Waste-Free Steps: Modular Synthesis of Biologically Active Lignin-Derived Scaffolds. ACS Cent. Sci. 2019, 5, 1707–1716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caetano, J.A.T.; Fernandes, A.C. One-pot Synthesis of Amines from Biomass Resources Catalyzed by HReO4. Green Chem. 2018, 20, 2494–2498. [Google Scholar] [CrossRef]
- Isca, V.M.S.; Fernandes, A.C. Direct Synthesis of α-Aminophosphonates from Biomass Resources Catalyzed by HReO4. Green Chem. 2018, 20, 3242–3245. [Google Scholar] [CrossRef]
- Jia, L.L.; Zhang, Z.; Qiao, Y.; Pedersen, C.M.; Ge, H.; Wei, Z.; Deng, T.; Ren, J.; Liu, X.; Wang, Y.; et al. Product Distribution Control for Glucosamine Condensation: Nuclear Magnetic Resonance (NMR) Investigation Substantiated by Density Functional Calculations. Ind. Eng. Chem. Res. 2017, 56, 2925–2934. [Google Scholar] [CrossRef]
- Song, L.; Zheng, M.; Pang, J.; Sebastian, J.; Wang, W.; Qu, M.; Zhao, J.; Wang, X.; Zhang, T. One-pot Synthesis of 2-Hydroxymethyl-5-methylpyrazine from Renewable 1,3-Dihydroxyacetone. Green Chem. 2017, 19, 3515–3519. [Google Scholar] [CrossRef]
- Chen, X.; Chew, S.L.; Kerton, F.M.; Yan, N. Direct Conversion of Chitin into a N-containing Furan Derivative. Green Chem. 2014, 16, 2204–2212. [Google Scholar] [CrossRef] [Green Version]
- Buggia, I.; Locatelli, F.; Regazzi, M.B.; Zecca, M. Busulfan. Ann. Pharm. 1994, 28, 1055–1062. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, T.; Hiraku, Y.; Oikawa, S.; Mizutani, H.; Kojima, M.; Kawanishi, S. DNA Intrastrand Cross-link at the 5′-GA-3′ Sequence Formed by Busulfan and its Role in the Cytotoxic Effect. Cancer Sci. 2004, 95, 454–458. [Google Scholar] [CrossRef] [Green Version]
- Krivoy, N.; Hoffer, E.; Lurie, Y.; Bentur, Y.; Rowe, M. Busulfan Use in Hematopoietic Stem Cell Transplantation: Pharmacology, Dose Adjustment, Safety and Efficacy in Adults and Children. Curr. Drug Saf. 2008, 3, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Myers, A.L.; Kawedia, J.D.; Champlin, R.E.; Kramer, M.A.; Nieto, Y.; Ghose, R.; Andersson, B.S. Clarifying Busulfan Metabolism and Drug Interactions to Support New Therapeutic Drug Monitoring Strategies: A Comprehensive Review. Expert Opin. Drug Metab. Toxicol. 2017, 13, 901–923. [Google Scholar] [CrossRef] [PubMed]
- Shiri, P.; Ramezanpour, S.; Amani, A.M.; Dehaen, W. A patent review on efficient strategies for the total synthesis of pazopanib, regorafenib and lenvatinib as novel anti-angiogenesis receptor tyrosine kinase inhibitors for cancer therapy. Mol. Divers. 2022, 1–22. [Google Scholar] [CrossRef]
- Wang, L.; Li, R.; Song, C.; Chen, Y.; Long, H.; Yang, L. Small-Molecule Anti-Cancer Drugs From 2016 to 2020: Synthesis and Clinical Application. Nat. Prod. Commun. 2021, 16, 1–42. [Google Scholar] [CrossRef]
- Noronha, R.G.; Fernandes, A.C. High Valent Oxo-Molybdenum Complexes as Efficient Catalysts for C-X Bond Forming Reactions. Curr. Org. Chem. 2012, 16, 33–64. [Google Scholar] [CrossRef]
- Pereira, J.G.; Sousa, S.C.A.; Fernandes, A.C. Direct Conversion of Carbohydrates into 5-Ethoxymethylfurfural (EMF) and 5-Hydroxymethylfurfural (HMF) Catalyzed by Oxomolybdenum Complexes. ChemistrySelect 2017, 2, 4516–4521. [Google Scholar] [CrossRef]
- Sousa, S.C.A.; Fernandes, T.A.; Fernandes, A.C. Highly Efficient Deoxygenation of Aryl Ketones to Arylalkanes Catalyzed by Dioxidomolybdenum Complexes. Eur. J. Org. Chem. 2016, 2016, 3109–3112. [Google Scholar] [CrossRef]
- Fernandes, T.A.; Fernandes, A.C. Dioxomolybdenum Complexes as Excellent Catalysts for the Deoxygenation of Aryl Ketones to Aryl Alkenes. ChemCatChem 2015, 7, 3503–3507. [Google Scholar] [CrossRef]
- Sousa, S.C.A.; Bernardo, J.R.; Wolff, M.; Machura, B.; Fernandes, A.C. Oxo-Rhenium(V) Complexes Containing Heterocycling Ligands as Catalysts for the Reduction of Sulfoxides. Eur. J. Org. Chem. 2014, 2014, 1855–1859. [Google Scholar] [CrossRef]
- Kobylarski, M.; Berthet, J.-C.; Cantat, T. Reductive Depolymerization of Polyesters and Polycarbonates with Hydroboranes by Using a Lanthanum(III) tris(amide) Catalyst. Chem. Commun. 2022, 58, 2830–2833. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Salas, J.A.; Manzini, S.; Nolan, S.P. Facile and efficient KOH-Catalysed Reduction of Esters and Tertiary Amides. Chem. Commun. 2013, 49, 9758–9760. [Google Scholar] [CrossRef] [PubMed]
- Arnáiz, F.J.; Aguado, R.; Pedrosa, M.R.; De Cian, A. Addition compounds of dichlorodioxomolybdenum(VI) with sulfoxides. Molecular structure of [MoO2Cl2(Me2SO)2]. Inorg. Chim. Acta 2003, 347, 33. [Google Scholar] [CrossRef]







| Entry | Catalyst (mol%) | Silane | Silane (Equiv.) | Temp. (°C) | Time (h) | Yield (%) b |
|---|---|---|---|---|---|---|
| 1 | 2 | PMHS | 1 | 110 | 24 | 39 |
| 2 | 2 | PMHS | 3 | 110 | 24 | 59 |
| 3 | 5 | PMHS | 3 | 110 | 24 | 63 |
| 4 | 2 | (EtO)2MeSiH | 3 | 110 | 24 | 50 |
| 5 | 5 | PhSiH3 | 3 | 110 | 24 | 55 |
| 6 | 5 | TMDS | 3 | 110 | 24 | 72 |
| 7 | 2 | TMDS | 2 | 110 | 24 | 52 |
| 8 | 2 | TMDS | 3 | 110 | 24 | 59 |
| 9 | 5 | TMDS | 2 | 110 | 24 | 52 |
| 10 | 5 | TMDS | 3 | r. t. | 48 | No reaction |
| 11 | 5 | TMDS | 3 | 110 | 24 | 65 c |

| Entry | Silane | Silane (Equiv.) | Temp. (°C) | Yield (%) b |
|---|---|---|---|---|
| 1 | PMHS | 2 | 110 | 48 |
| 2 | PMHS | 6 | 110 | 72 |
| 3 | PhSiH3 | 6 | 110 | 67 |
| 4 | TMDS | 6 | 110 | 75 |
| 5 | TMDS | 4 | 110 | 48 |
| 6 | TMDS | 6 | r. t. | No reaction |
| 7 | TMDS | 6 | 110 | 69 c |

| Entry | Polyester | KOH (Equiv.) | Silane | Silane (Equiv.) | Temperature (°C) | Time (h) | Yield (%) b |
|---|---|---|---|---|---|---|---|
| 1 | P4HB | 0.4 | PhSiH3 | 3 | 110 | 24 | 95 |
| 2 | P4HB | 0.4 | PhSiH3 | 2 | 110 | 24 | 38 |
| 3 | P4HB | 0.4 | PhSiH3 | 3 | r. t. | 24 | No reaction |
| 4 | P4HB | 0.4 | TMDS | 3 | 110 | 24 | 65 |
| 5 | P4HB | 0.4 | PMHS | 3 | 110 | 48 | 47 |
| 6 | PBS | 0.8 | PhSiH3 | 6 | 110 | 48 | 61c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lourenço, D.L.; Fernandes, A.C. Depolymerization of P4HB and PBS Waste and Synthesis of the Anticancer Drug Busulfan from Plastic Waste. Catalysts 2022, 12, 381. https://doi.org/10.3390/catal12040381
Lourenço DL, Fernandes AC. Depolymerization of P4HB and PBS Waste and Synthesis of the Anticancer Drug Busulfan from Plastic Waste. Catalysts. 2022; 12(4):381. https://doi.org/10.3390/catal12040381
Chicago/Turabian StyleLourenço, Daniel L., and Ana C. Fernandes. 2022. "Depolymerization of P4HB and PBS Waste and Synthesis of the Anticancer Drug Busulfan from Plastic Waste" Catalysts 12, no. 4: 381. https://doi.org/10.3390/catal12040381