Temperature-Dependent Activity of Gold Nanocatalysts Supported on Activated Carbon in Redox Catalytic Reactions: 5-Hydroxymethylfurfural Oxidation and 4-Nitrophenol Reduction Comparison
Abstract
:1. Introduction
2. Results
2.1. Characterization of Catalysts
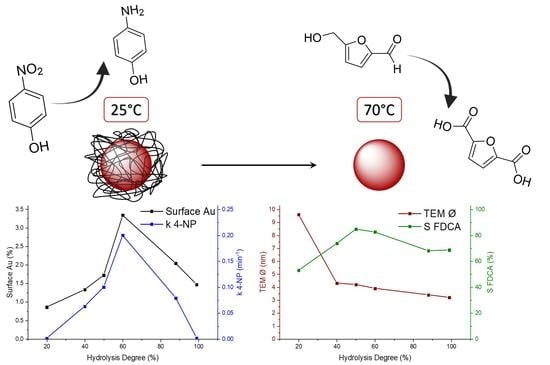
2.2. Catalytic Tests on HMF Oxidation
2.3. Catalytic Tests on 4-Nitrophenol Reduction
2.4. Characterization of Au Colloidal Nanoparticles for Evaluating the Difference in Catalytic Activity of the Two Reactions Presented
2.5. Effect of Polymer Removal
2.6. Titania-Supported Catalysts
2.7. Characterization of the Washed Catalysts
3. Materials and Methods
3.1. Materials
3.2. Catalysts Preparation
3.3. Characterization
3.4. Catalytic Tests
3.4.1. 5-Hydroxymethylfurfural Oxidation Test
3.4.2. 4-Nitrophenol Calibration Test
3.4.3. Catalytic Reduction of 4-Nitrophenol
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rogers, C.; Perkins, W.S.; Veber, G.; Williams, T.E.; Cloke, R.R.; Fischer, F.R. Synergistic Enhancement of Electrocatalytic CO2 Reduction with Gold Nanoparticles Embedded in Functional Graphene Nanoribbon Composite Electrodes. J. Am. Chem. Soc. 2017, 139, 4052–4061. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torres Galvis, H.M.; Bitter, J.H.; Khare, C.B.; Ruitenbeek, M.; Dugulan, A.I.; de Jong, K.P. Supported Iron Nanoparticles as Catalysts for Sustainable Production of Lower Olefins. Science 2012, 335, 835–838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gandarias, I.; Miedziak, P.J.; Nowicka, E.; Douthwaite, M.; Morgan, D.J.; Hutchings, G.J.; Taylor, S.H. Selective Oxidation of N-Butanol Using Gold-Palladium Supported Nanoparticles Under Base-Free Conditions. ChemSusChem 2015, 8, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Tada, H. Overall Water Splitting and Hydrogen Peroxide Synthesis by Gold Nanoparticle-Based Plasmonic Photocatalysts. Nanoscale Adv. 2019, 1, 4238–4245. [Google Scholar] [CrossRef] [Green Version]
- Whipple, D.T.; Kenis, P.J.A. Prospects of CO2 Utilization via Direct Heterogeneous Electrochemical Reduction. J. Phys. Chem. Lett. 2010, 1, 3451–3458. [Google Scholar] [CrossRef]
- Hall, A.S.; Yoon, Y.; Wuttig, A.; Surendranath, Y. Mesostructure-Induced Selectivity in CO2 Reduction Catalysis. J. Am. Chem. Soc. 2015, 137, 14834–14837. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Wang, C.; Sun, H.-L.; Fu, G.; Chen, S.; Zhang, Y.-J.; Chen, B.-H.; Anema, J.R.; Yang, Z.-L.; Li, J.-F.; et al. In Situ Dynamic Tracking of Heterogeneous Nanocatalytic Processes by Shell-Isolated Nanoparticle-Enhanced Raman Spectroscopy. Nat. Commun. 2017, 8, 15447. [Google Scholar] [CrossRef] [Green Version]
- Hafeez, S.; Sanchez, F.; Al-Salem, S.M.; Villa, A.; Manos, G.; Dimitratos, N.; Constantinou, A. Decomposition of Additive-Free Formic Acid Using a Pd/C Catalyst in Flow: Experimental and CFD Modelling Studies. Catalysts 2021, 11, 341. [Google Scholar] [CrossRef]
- Ndolomingo, M.J.; Bingwa, N.; Meijboom, R. Review of Supported Metal Nanoparticles: Synthesis Methodologies, Advantages and Application as Catalysts. J. Mater. Sci. 2020, 55, 6195–6241. [Google Scholar] [CrossRef]
- Hsieh, B.-J.; Tsai, M.-C.; Pan, C.-J.; Su, W.-N.; Rick, J.; Chou, H.-L.; Lee, J.-F.; Hwang, B.-J. Tuning Metal Support Interactions Enhances the Activity and Durability of TiO2-Supported Pt Nanocatalysts. Electrochim. Acta 2017, 224, 452–459. [Google Scholar] [CrossRef]
- Chen, S.; Abdel-Mageed, A.M.; Gauckler, C.; Olesen, S.E.; Chorkendorff, I.; Behm, R.J. Selective CO Methanation on Isostructural Ru Nanocatalysts: The Role of Support Effects. J. Catal. 2019, 373, 103–115. [Google Scholar] [CrossRef]
- Ferrando, R.; Jellinek, J.; Johnston, R.L. Nanoalloys: From Theory to Applications of Alloy Clusters and Nanoparticles. Chem. Rev. 2008, 108, 845–910. [Google Scholar] [CrossRef] [PubMed]
- Iben Ayad, A.; Luart, D.; Ould Dris, A.; Guénin, E. Kinetic Analysis of 4-Nitrophenol Reduction by “Water-Soluble” Palladium Nanoparticles. Nanomaterials 2020, 10, 1169. [Google Scholar] [CrossRef]
- Daraee, H.; Eatemadi, A.; Abbasi, E.; Fekri Aval, S.; Kouhi, M.; Akbarzadeh, A. Application of Gold Nanoparticles in Biomedical and Drug Delivery. Artif. Cells Nanomed. Biotechnol. 2016, 44, 410–422. [Google Scholar] [CrossRef]
- Bingwa, N.; Patala, R.; Noh, J.-H.; Ndolomingo, M.J.; Tetyana, S.; Bewana, S.; Meijboom, R. Synergistic Effects of Gold–Palladium Nanoalloys and Reducible Supports on the Catalytic Reduction of 4-Nitrophenol. Langmuir 2017, 33, 7086–7095. [Google Scholar] [CrossRef]
- Vial, S.; Reis, R.L.; Oliveira, J.M. Recent Advances Using Gold Nanoparticles as a Promising Multimodal Tool for Tissue Engineering and Regenerative Medicine. Curr. Opin. Solid State Mater. Sci. 2017, 21, 92–112. [Google Scholar] [CrossRef] [Green Version]
- Jin, L.; Liu, B.; Duay, S.; He, J. Engineering Surface Ligands of Noble Metal Nanocatalysts in Tuning the Product Selectivity. Catalysts 2017, 7, 44. [Google Scholar] [CrossRef] [Green Version]
- Narayanan, R.; El-Sayed, M.A. Shape-Dependent Catalytic Activity of Platinum Nanoparticles in Colloidal Solution. Nano Lett. 2004, 4, 1343–1348. [Google Scholar] [CrossRef]
- Tian, N.; Zhou, Z.-Y.; Sun, S.-G.; Ding, Y.; Wang, Z.L. Synthesis of Tetrahexahedral Platinum Nanocrystals with High-Index Facets and High Electro-Oxidation Activity. Science 2007, 316, 732–735. [Google Scholar] [CrossRef]
- Nanda, K.K. Size-Dependent Density of Nanoparticles and Nanostructured Materials. Phys. Lett. A 2012, 376, 3301–3302. [Google Scholar] [CrossRef]
- Lim, D.C.; Lopez-Salido, I.; Dietsche, R.; Bubek, M.; Kim, Y.D. Size-Selectivity in the Oxidation Behaviors of Au Nanoparticles. Angew. Chem. Int. Ed. 2006, 45, 2413–2415. [Google Scholar] [CrossRef] [PubMed]
- Megías-Sayago, C.; Lolli, A.; Bonincontro, D.; Penkova, A.; Albonetti, S.; Cavani, F.; Odriozola, J.A.; Ivanova, S. Effect of Gold Particles Size over Au/C Catalyst Selectivity in HMF Oxidation Reaction. ChemCatChem 2020, 12, 1177–1183. [Google Scholar] [CrossRef]
- Fritz, G.; Schädler, V.; Willenbacher, N.; Wagner, N.J. Electrosteric Stabilization of Colloidal Dispersions. Langmuir 2002, 18, 6381–6390. [Google Scholar] [CrossRef]
- Polte, J. Fundamental Growth Principles of Colloidal Metal Nanoparticles—A New Perspective. CrystEngComm 2015, 17, 6809–6830. [Google Scholar] [CrossRef] [Green Version]
- Rossi, L.M.; Fiorio, J.L.; Garcia, M.A.S.; Ferraz, C.P. The Role and Fate of Capping Ligands in Colloidally Prepared Metal Nanoparticle Catalysts. Dalton Trans. 2018, 47, 5889–5915. [Google Scholar] [CrossRef]
- Ansar, S.M.; Kitchens, C.L. Impact of Gold Nanoparticle Stabilizing Ligands on the Colloidal Catalytic Reduction of 4-Nitrophenol. ACS Catal. 2016, 6, 5553–5560. [Google Scholar] [CrossRef]
- Zhao, Y.; Baeza, J.A.; Koteswara Rao, N.; Calvo, L.; Gilarranz, M.A.; Li, Y.D.; Lefferts, L. Unsupported PVA- and PVP-Stabilized Pd Nanoparticles as Catalyst for Nitrite Hydrogenation in Aqueous Phase. J. Catal. 2014, 318, 162–169. [Google Scholar] [CrossRef]
- Thiele, H.; von Levern, H.S. Synthetic Protective Colloids. J. Colloid Sci. 1965, 20, 679–694. [Google Scholar] [CrossRef]
- Singh, M.; Sinha, I.; Premkumar, M.; Singh, A.K.; Mandal, R.K. Structural and Surface Plasmon Behavior of Cu Nanoparticles Using Different Stabilizers. Colloids Surf. A Physicochem. Eng. Asp. 2010, 359, 88–94. [Google Scholar] [CrossRef]
- Abis, L.; Dimitritatos, N.; Sankar, M.; Freakley, S.J.; Hutchings, G.J. The Effect of Polymer Addition on Base Catalyzed Glycerol Oxidation Using Gold and Gold–Palladium Bimetallic Catalysts. Top. Catal. 2020, 63, 394–402. [Google Scholar] [CrossRef] [Green Version]
- Kim, E.J.; Yeum, J.H.; Choi, J.H. Effects of Polymeric Stabilizers on the Synthesis of Gold Nanoparticles. J. Mater. Sci. Technol. 2014, 30, 107–111. [Google Scholar] [CrossRef]
- Chen, G.; Xu, C.; Huang, X.; Ye, J.; Gu, L.; Li, G.; Tang, Z.; Wu, B.; Yang, H.; Zhao, Z.; et al. Interfacial Electronic Effects Control the Reaction Selectivity of Platinum Catalysts. Nat. Mater. 2016, 15, 564–569. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Sham, T.K. Tuning the Electronic Behavior of Au Nanoparticles with Capping Molecules. Appl. Phys. Lett. 2002, 81, 736–738. [Google Scholar] [CrossRef]
- Schäfer, C.; Mhadgut, S.C.; Kugyela, N.; Török, M.; Török, B. Proline-Induced Enantioselective Heterogeneous Catalytic Hydrogenation of Isophorone on Basic Polymer-Supported Pd Catalysts. Catal. Sci. Technol. 2015, 5, 716–723. [Google Scholar] [CrossRef]
- Liu, J.; Bai, P.; Zhao, X.S. Ruthenium Nanoparticles Embedded in Mesoporous Carbon Microfibers: Preparation, Characterization and Catalytic Properties in the Hydrogenation of d-Glucose. Phys. Chem. Chem. Phys. 2011, 13, 3758–3763. [Google Scholar] [CrossRef]
- Albonetti, S.; Lolli, A.; Morandi, V.; Migliori, A.; Lucarelli, C.; Cavani, F. Conversion of 5-Hydroxymethylfurfural to 2,5-Furandicarboxylic Acid over Au-Based Catalysts: Optimization of Active Phase and Metal–Support Interaction. Appl. Catal. B Environ. 2015, 163, 520–530. [Google Scholar] [CrossRef]
- Lolli, A.; Albonetti, S.; Utili, L.; Amadori, R.; Ospitali, F.; Lucarelli, C.; Cavani, F. Insights into the Reaction Mechanism for 5-Hydroxymethylfurfural Oxidation to FDCA on Bimetallic Pd–Au Nanoparticles. Appl. Catal. A Gen. 2015, 504, 408–419. [Google Scholar] [CrossRef]
- Li, X.; Xu, J.; Wang, F.; Gao, J.; Zhou, L.; Yang, G. Direct Oxidation of Toluene to Benzoic Acid with Molecular Oxygen over Manganese Oxides. Catal. Lett. 2006, 108, 137–140. [Google Scholar] [CrossRef]
- Davis, S.E.; Houk, L.R.; Tamargo, E.C.; Datye, A.K.; Davis, R.J. Oxidation of 5-Hydroxymethylfurfural over Supported Pt, Pd and Au Catalysts. Catal. Today 2011, 160, 55–60. [Google Scholar] [CrossRef]
- Van Putten, R.-J.; van der Waal, J.C.; de Jong, E.; Rasrendra, C.B.; Heeres, H.J.; de Vries, J.G. Hydroxymethylfurfural, A Versatile Platform Chemical Made from Renewable Resources. Chem. Rev. 2013, 113, 1499–1597. [Google Scholar] [CrossRef]
- Scurti, S.; Monti, E.; Rodríguez-Aguado, E.; Caretti, D.; Cecilia, J.A.; Dimitratos, N. Effect of Polyvinyl Alcohol Ligands on Supported Gold Nano-Catalysts: Morphological and Kinetics Studies. Nanomaterials 2021, 11, 879. [Google Scholar] [CrossRef] [PubMed]
- Albonetti, S.; Pasini, T.; Lolli, A.; Blosi, M.; Piccinini, M.; Dimitratos, N.; Lopez-Sanchez, J.A.; Morgan, D.J.; Carley, A.F.; Hutchings, G.J.; et al. Selective Oxidation of 5-Hydroxymethyl-2-Furfural over TiO2-Supported Gold–Copper Catalysts Prepared from Preformed Nanoparticles: Effect of Au/Cu Ratio. Catal. Today 2012, 195, 120–126. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, G.; Guay, D.; Chaker, M.; Ma, D. Highly Active PtAu Alloy Nanoparticle Catalysts for the Reduction of 4-Nitrophenol. Nanoscale 2014, 6, 2125–2130. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.; Qu, Y.; Pei, X.; Li, S.; You, S.; Wang, J.; Zhang, Z.; Zhou, J. Catalytic Reduction of 4-Nitrophenol Using Gold Nanoparticles Biosynthesized by Cell-Free Extracts of Aspergillus Sp. WL-Au. J. Hazard. Mater. 2017, 321, 299–306. [Google Scholar] [CrossRef]
- Aditya, T.; Jana, J.; Singh, N.K.; Pal, A.; Pal, T. Remarkable Facet Selective Reduction of 4-Nitrophenol by Morphologically Tailored (111) Faceted Cu2O Nanocatalyst. ACS Omega 2017, 2, 1968–1984. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wunder, S.; Polzer, F.; Lu, Y.; Mei, Y.; Ballauff, M. Kinetic Analysis of Catalytic Reduction of 4-Nitrophenol by Metallic Nanoparticles Immobilized in Spherical Polyelectrolyte Brushes. J. Phys. Chem. C 2010, 114, 8814–8820. [Google Scholar] [CrossRef]
- Gu, S.; Wunder, S.; Lu, Y.; Ballauff, M.; Fenger, R.; Rademann, K.; Jaquet, B.; Zaccone, A. Kinetic Analysis of the Catalytic Reduction of 4-Nitrophenol by Metallic Nanoparticles. J. Phys. Chem. C 2014, 118, 18618–18625. [Google Scholar] [CrossRef]
- Lopez-Sanchez, J.A.; Dimitratos, N.; Hammond, C.; Brett, G.L.; Kesavan, L.; White, S.; Miedziak, P.; Tiruvalam, R.; Jenkins, R.L.; Carley, A.F.; et al. Facile Removal of Stabilizer-Ligands from Supported Gold Nanoparticles. Nat. Chem. 2011, 3, 551–556. [Google Scholar] [CrossRef]
- Baker, L.R.; Kennedy, G.; Krier, J.M.; Van Spronsen, M.; Onorato, R.M.; Somorjai, G.A. The Role of an Organic Cap in Nanoparticle Catalysis: Reversible Restructuring of Carbonaceous Material Controls Catalytic Activity of Platinum Nanoparticles for Ethylene Hydrogenation and Methanol Oxidation. Catal. Lett. 2012, 142, 1286–1294. [Google Scholar] [CrossRef]
- Yang, N.; Pattisson, S.; Douthwaite, M.; Zeng, G.; Zhang, H.; Ma, J.; Hutchings, G.J. Influence of Stabilizers on the Performance of Au/TiO 2 Catalysts for CO Oxidation. ACS Catal. 2021, 11, 11607–11615. [Google Scholar] [CrossRef]
- Zielińska-Jurek, A.; Kowalska, E.; Sobczak, J.W.; Lisowski, W.; Ohtani, B.; Zaleska, A. Preparation and Characterization of Monometallic (Au) and Bimetallic (Ag/Au) Modified-Titania Photocatalysts Activated by Visible Light. Appl. Catal. B Environ. 2011, 101, 504–514. [Google Scholar] [CrossRef]
- Chen, M.; Goodman, D.W. Catalytically Active Gold: From Nanoparticles to Ultrathin Films. Acc. Chem. Res. 2006, 39, 739–746. [Google Scholar] [CrossRef] [PubMed]
- Moma, J.A.; Scurrell, M.S.; Jordaan, W.A. Effects of Incorporation of Ions into Au/TiO2 Catalysts for Carbon Monoxide Oxidation. Top. Catal. 2007, 44, 167–172. [Google Scholar] [CrossRef]
- Mohite, V.S.; Mahadik, M.A.; Kumbhar, S.S.; Hunge, Y.M.; Kim, J.H.; Moholkar, A.V.; Rajpure, K.Y.; Bhosale, C.H. Photoelectrocatalytic Degradation of Benzoic Acid Using Au Doped TiO2 Thin Films. J. Photochem. Photobiol. B Biol. 2015, 142, 204–211. [Google Scholar] [CrossRef]
- Yu, J.C.; Yu, J.; Zhao, J. Enhanced Photocatalytic Activity of Mesoporous and Ordinary TiO2 Thin Films by Sulfuric Acid Treatment. Appl. Catal. B Environ. 2002, 36, 31–43. [Google Scholar] [CrossRef]
- Bowers, G.N.; McComb, R.B.; Christensen, R.C.; Schaffer, R. High-Purity 4-Nitrophenol: Purification, Characterization, and Specifications for Use as a Spectrophotometric Reference Material. Clin. Chem. 1980, 26, 724–729. [Google Scholar] [CrossRef]
- Kumar, A.; Belwal, M.; Maurya, R.R.; Mohan, V.; Vishwanathan, V. Heterogeneous Catalytic Reduction of Anthropogenic Pollutant, 4-Nitrophenol by Au/AC Nanocatalysts. Mater. Sci. Energy Technol. 2019, 2, 526–531. [Google Scholar] [CrossRef]















| Sample | PVA | PVA HD (%) | TEM Ø (nm) | Surface Au (at%) |
|---|---|---|---|---|
| Au/AC_PVA-20 | Synthesized | 20 | 9.6 | 0.86 |
| Au/AC_PVA-40 | Synthesized | 40 | 4.3 | 1.33 |
| Au/AC_PVA-50 | Synthesized | 50 | 4.2 | 1.72 |
| Au/AC_PVA-60 | Synthesized | 60 | 3.9 | 3.34 |
| Au/AC_PVA-88 | Commercial | 88 | 3.4 | 2.04 |
| Au/AC_PVA-99 | Commercial | 99 | 3.2 | 1.47 |
| 4-NP (25 °C) | HMF (70 °C) | |||||
|---|---|---|---|---|---|---|
| Sample | kapp (min−1) | X4-NP (%) | X HMF (%) | S HMFCA (%) | S FFCA (%) | S FDCA (%) |
| Au/AC_PVA-99 | 1.8 × 10−3 ± 4 × 10−4 | 16 ± 0.2 | 100 | 31 | 0 | 69 |
| Au/AC_PVA-99-W | 0.3 ± 3 × 10−2 | 99 ± 1 | 100 | 26 | 0 | 74 |
| Au/TiO2_PVA-99 | 2.5 × 10−2 ± 1 × 10−3 | 48 ± 1 | 100 | 59 | 1 | 40 |
| Au/TiO2_PVA-99-W | 0.1 ± 3 × 10−3 | 99 ± 1 | 100 | 54 | 1 | 45 |
| Sample | BE Au 4f 7/2 (eV) | Surface Au (%) | Surface C (%) | Surface Ti (%) | Au/Ti |
|---|---|---|---|---|---|
| Au/AC_PVA-99 | 84.1 | 1.47 | 91.2 | - | 0.02 |
| Au/AC_PVA-99-w | 84.1 | 2.34 | 88.2 | - | 0.03 |
| Au/TiO2_PVA-99 | 83.2 | 0.68 | 22.0 | 22.7 | 0.03 |
| Au/TiO2_PVA-99-w | 83.2 | 1.26 | 22.0 | 22.0 | 0.06 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scurti, S.; Allegri, A.; Liuzzi, F.; Rodríguez-Aguado, E.; Cecilia, J.A.; Albonetti, S.; Caretti, D.; Dimitratos, N. Temperature-Dependent Activity of Gold Nanocatalysts Supported on Activated Carbon in Redox Catalytic Reactions: 5-Hydroxymethylfurfural Oxidation and 4-Nitrophenol Reduction Comparison. Catalysts 2022, 12, 323. https://doi.org/10.3390/catal12030323
Scurti S, Allegri A, Liuzzi F, Rodríguez-Aguado E, Cecilia JA, Albonetti S, Caretti D, Dimitratos N. Temperature-Dependent Activity of Gold Nanocatalysts Supported on Activated Carbon in Redox Catalytic Reactions: 5-Hydroxymethylfurfural Oxidation and 4-Nitrophenol Reduction Comparison. Catalysts. 2022; 12(3):323. https://doi.org/10.3390/catal12030323
Chicago/Turabian StyleScurti, Stefano, Alessandro Allegri, Francesca Liuzzi, Elena Rodríguez-Aguado, Juan Antonio Cecilia, Stefania Albonetti, Daniele Caretti, and Nikolaos Dimitratos. 2022. "Temperature-Dependent Activity of Gold Nanocatalysts Supported on Activated Carbon in Redox Catalytic Reactions: 5-Hydroxymethylfurfural Oxidation and 4-Nitrophenol Reduction Comparison" Catalysts 12, no. 3: 323. https://doi.org/10.3390/catal12030323