Wavelength Dependence of the Photocatalytic Performance of Pure and Doped TiO2 Photocatalysts—A Reflection on the Importance of UV Excitability
Abstract
:1. Introduction
2. Results and Discussion
2.1. Photocatalytic Performance
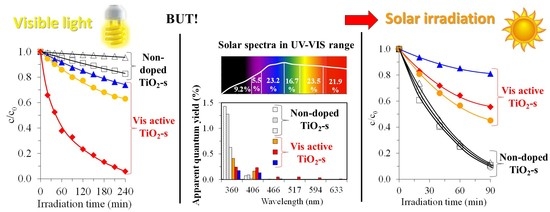
2.1.1. Experiments with Visible-Light-Emitting Energy-Saving Compact Fluorescence Lamps
2.1.2. Solar Experiments
2.1.3. Photocatalytic Experiments with UV Lights and Different Colored LED Lights
2.2. Incident Photon Fluxes
2.3. Discussion of the Calculated Apparent Quantum Yields
3. Materials and Methods
3.1. Materials
3.2. Methods and Instrumentation
3.2.1. Photocatalytic Experiments
3.2.2. Potassium Ferrioxalate Actinometry
3.2.3. Light Intensity Measurements beyond the Ferrioxalate Method Validity Interval
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Menéndez-Flores, V.M.; Bahnemann, D.W.; Ohno, T. Visible light photocatalytic activities of S-doped TiO2-Fe3+ in aqueous and gas phase. Appl. Catal. B 2011, 103, 99–108. [Google Scholar] [CrossRef]
- Li, X.; Liu, Y.; Yang, P.; Shi, Y. Visible light-driven photocatalysis of W, N co-doped TiO2. Particuology 2013, 11, 732–736. [Google Scholar] [CrossRef]
- Kumar, S.; Bhawna; Sharma, R.; Gupta, A.; Dubey, K.K.; Khan, A.M.; Singhal, R.; Kumar, R.; Bharti, A.; Singh, P.; et al. TiO2 based Photocatalysis membranes: An efficient strategy for pharmaceutical mineralization. Sci. Total Environ. 2022, 845, 157221. [Google Scholar] [CrossRef]
- Pelosato, R.; Bolognino, I.; Fontana, F.; Sora, I.N. Applications of Heterogeneous Photocatalysis to the Degradation of Oxytetracycline in Water: A Review. Molecules 2022, 27, 2743. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, A.; Muhmood, T.; Ahmad, F. Carbon nanotubes heterojunction with graphene like carbon nitride for the enhancement of electrochemical and photocatalytic activity. Mater. Chem. Phys. 2022, 278, 125640. [Google Scholar] [CrossRef]
- Muhmood, T.; Xia, M.; Lei, W.; Wang, F. Under vacuum synthesis of type-I heterojunction between red phosphorus and graphene like carbon nitride with enhanced catalytic, electrochemical and charge separation ability for photodegradation of an acute toxicity category-III compound. Appl. Catal. B 2018, 238, 568–575. [Google Scholar] [CrossRef]
- Santos, E.N.; Ágoston, Á.; Kertész, S.; Hodúr, C.; László, Z.; Pap, Z.; Kása, Z.; Alapi, T.; Krishnan, S.A.G.; Arthanareeswaran, G.; et al. Investigation of the applicability of TiO2, BiVO4, and WO3 nanomaterials for advanced photocatalytic membranes used for oil-in-water emulsion separation. Asia-Pac. J. Chem. Eng. 2020, 15, e2549. [Google Scholar]
- Nascimbén Santos, É.; László, Z.; Hodúr, C.; Arthanareeswaran, G.; Veréb, G. Photocatalytic membrane filtration and its advantages over conventional approaches in the treatment of oily wastewater: A review. Asia-Pac. J. Chem. Eng. 2020, 15, e2533. [Google Scholar] [CrossRef]
- Zarghami, S.; Mohammadi, T.; Sadrzadeh, M.; Van der Bruggen, B. Superhydrophilic and underwater superoleophobic membranes—A review of synthesis methods. Prog. Polym. Sci. 2019, 98, 101166. [Google Scholar] [CrossRef]
- Mogyorosi, K.; Kmetyko, A.; Czirbus, N.; Vereb, G.; Sipos, P.; Dombi, A. Comparison of the substrate dependent performance of Pt-, Au- and Ag-doped TiO(2) photocatalysts in H(2)-production and in decomposition of various organics. React. Kinet. Catal. Lett. 2009, 98, 215–225. [Google Scholar] [CrossRef]
- Escobedo, S.; de Lasa, H. Synthesis and Performance of Photocatalysts for Photocatalytic Hydrogen Production: Future Perspectives. Catalysts 2021, 11, 1505. [Google Scholar] [CrossRef]
- Corredor, J.; Rivero, M.J.; Rangel, C.M.; Gloaguen, F.; Ortiz, I. Comprehensive review and future perspectives on the photocatalytic hydrogen production. J. Chem. Technol. Biotechnol. 2019, 94, 3049–3063. [Google Scholar] [CrossRef] [Green Version]
- Hu, J.D.; Cao, Y.L.; Wang, K.; Jia, D.Z. Green solid-state synthesis and photocatalytic hydrogen production activity of anatase TiO2 nanoplates with super heat-stability. RSC Adv. 2017, 7, 11827–11833. [Google Scholar] [CrossRef] [Green Version]
- Bojinova, A.; Kaneva, N.; Papazova, K.; Eliyas, A.; Stoyanova-Eliyas, E.; Dimitrov, D. Green synthesis of UV and visible light active TiO2/WO3 powders and films for malachite green and ethylene photodegradation. React. Kinet. Mech. Catal. 2017, 120, 821–832. [Google Scholar] [CrossRef]
- Serpone, N. Heterogeneous Photocatalysis and Prospects of TiO2-Based Photocatalytic DeNOxing the Atmospheric Environment. Catalysts 2018, 8, 553. [Google Scholar] [CrossRef] [Green Version]
- Wu, D.; Wang, L.; Song, X.; Tan, Y. Enhancing the visible-light-induced photocatalytic activity of the self-cleaning TiO2-coated cotton by loading Ag/AgCl nanoparticles. Thin Solid Films 2013, 540, 36–40. [Google Scholar] [CrossRef]
- Soklič, A.; Tasbihi, M.; Kete, M.; Štangar, U.L. Deposition and possible influence of a self-cleaning thin TiO2/SiO2 film on a photovoltaic module efficiency. Catal. Today 2015, 252, 54–60. [Google Scholar] [CrossRef]
- Tallósy, S.P.; Janovák, L.; Ménesi, J.; Nagy, E.; Juhász, Á.; Balázs, L.; Deme, I.; Buzás, N.; Dékány, I. Investigation of the antibacterial effects of silver-modified TiO2 and ZnO plasmonic photocatalysts embedded in polymer thin films. Environ. Sci. Pollut. Res. 2014, 21, 11155–11167. [Google Scholar] [CrossRef]
- Arunachalam, P.; Nagai, K.; Amer, M.S.; Ghanem, M.A.; Ramalingam, R.J.; Al-Mayouf, A.M. Recent Developments in the Use of Heterogeneous Semiconductor Photocatalyst Based Materials for a Visible-Light-Induced Water-Splitting System—A Brief Review. Catalysts 2021, 11, 160. [Google Scholar] [CrossRef]
- Ma, J.; Chu, L.; Guo, Y.; Sun, C.; Yan, H.; Li, Z.; Li, M. Graphene Quantum Dots Improved “Caterpillar”-like TiO2 for Highly Efficient Photocatalytic Hydrogen Production. Materials 2021, 14, 5354. [Google Scholar] [CrossRef]
- Nguyen, V.-H.; Nguyen, B.-S.; Jin, Z.; Shokouhimehr, M.; Jang, H.W.; Hu, C.; Singh, P.; Raizada, P.; Peng, W.; Shiung Lam, S.; et al. Towards artificial photosynthesis: Sustainable hydrogen utilization for photocatalytic reduction of CO2 to high-value renewable fuels. Chem. Eng. J. 2020, 402, 126184. [Google Scholar] [CrossRef]
- He, J.; Janaky, C. Recent Advances in Solar-Driven Carbon Dioxide Conversion: Expectations versus Reality. ACS Energy Lett. 2020, 5, 1996–2014. [Google Scholar] [CrossRef] [PubMed]
- Qamar, M.; Merzougui, B.; Anjum, D.; Hakeem, A.S.; Yamani, Z.H.; Bahnemann, D. Synthesis and photocatalytic activity of mesoporous nanocrystalline Fe-doped titanium dioxide. Catal. Today 2014, 230, 158–165. [Google Scholar] [CrossRef]
- Ansari, S.A.; Khan, M.M.; Ansari, M.O.; Cho, M.H. Nitrogen-doped titanium dioxide (N-doped TiO2) for visible light photocatalysis. New J. Chem. 2016, 40, 3000–3009. [Google Scholar] [CrossRef]
- Li, Y.; Li, M.; Xu, P.; Tang, S.; Liu, C. Efficient photocatalytic degradation of acid orange 7 over N-doped ordered mesoporous titania on carbon fibers under visible-light irradiation based on three synergistic effects. Appl. Catal. A Gen. 2016, 524, 163–172. [Google Scholar] [CrossRef]
- Ahmed, S.; Rasul, M.G.; Brown, R.; Hashib, M.A. Influence of parameters on the heterogeneous photocatalytic degradation of pesticides and phenolic contaminants in wastewater: A short review. J. Environ. Manag. 2011, 92, 311–330. [Google Scholar] [CrossRef] [Green Version]
- Ohtani, B. Titania Photocatalysis beyond Recombination: A Critical Review. Catalysts 2013, 3, 942–953. [Google Scholar] [CrossRef] [Green Version]
- Nosaka, Y.; Nosaka, A.Y. Langmuir-Hinshelwood and Light-Intensity Dependence Analyses of Photocatalytic Oxidation Rates by Two-Dimensional-Ladder Kinetic Simulation. J. Phys. Chem. C 2018, 122, 28748–28756. [Google Scholar] [CrossRef]
- Nafradi, M.; Alapi, T.; Bencsik, G.; Janaky, C. Impact of Reaction Parameters and Water Matrices on the Removal of Organic Pollutants by TiO2/LED and ZnO/LED Heterogeneous Photocatalysis Using 365 and 398 nm Radiation. Nanomaterials 2021, 12, 5. [Google Scholar] [CrossRef]
- Nafradi, M.; Alapi, T.; Farkas, L.; Bencsik, G.; Kozma, G.; Hernadi, K. Wavelength Dependence of the Transformation Mechanism of Sulfonamides Using Different LED Light Sources and TiO2 and ZnO Photocatalysts. Materials 2021, 15, 49. [Google Scholar] [CrossRef]
- Banerjee, S.; Gopal, J.; Muraleedharan, P.; Tyagi, A.; Rai, B. Physics and chemistry of photocatalytic titanium dioxide: Visualization of bactericidal activity using atomic force microscopy. Curr. Sci. 2005, 90, 1378–1383. [Google Scholar]
- Rengifo-Herrera, J.A.; Pierzchała, K.; Sienkiewicz, A.; Forró, L.; Kiwi, J.; Pulgarin, C. Abatement of organics and Escherichia coli by N, S co-doped TiO2 under UV and visible light. Implications of the formation of singlet oxygen (1O2) under visible light. Appl. Catal. B 2009, 88, 398–406. [Google Scholar] [CrossRef]
- Iwase, M.; Yamada, K.; Kurisaki, T.; Prieto-Mahaney, O.O.; Ohtani, B.; Wakita, H. Visible-light photocatalysis with phosphorus-doped titanium(IV) oxide particles prepared using a phosphide compound. Appl. Catal. B 2013, 132–133, 39–44. [Google Scholar] [CrossRef]
- Myilsamy, M.; Murugesan, V.; Mahalakshmi, M. Indium and cerium co-doped mesoporous TiO2 nanocomposites with enhanced visible light photocatalytic activity. Appl. Catal. A Gen. 2015, 492, 212–222. [Google Scholar] [CrossRef]
- Nishiyama, N.; Kozasa, K.; Yamazaki, S. Photocatalytic degradation of 4-chlorophenol on titanium dioxide modified with Cu(II) or Cr(III) ion under visible light irradiation. Appl. Catal. A Gen. 2016, 527, 109–115. [Google Scholar] [CrossRef]
- Wu, P.; Xie, R.; Imlay, J.A.; Shang, J.K. Visible-light-induced photocatalytic inactivation of bacteria by composite photocatalysts of palladium oxide and nitrogen-doped titanium oxide. Appl. Catal. B 2009, 88, 576–581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rengifo-Herrera, J.A.; Kiwi, J.; Pulgarin, C. N, S co-doped and N-doped Degussa P-25 powders with visible light response prepared by mechanical mixing of thiourea and urea. Reactivity towards E. coli inactivation and phenol oxidation. J. Photochem. Photobiol. A 2009, 205, 109–115. [Google Scholar] [CrossRef]
- Rengifo-Herrera, J.A.; Pulgarin, C. Photocatalytic activity of N, S co-doped and N-doped commercial anatase TiO2 powders towards phenol oxidation and E. coli inactivation under simulated solar light irradiation. Solar Energy 2010, 84, 37–43. [Google Scholar] [CrossRef]
- Pap, Z.; Baia, L.; Mogyorosi, K.; Dombi, A.; Oszko, A.; Danciu, V. Correlating the visible light photoactivity of N-doped TiO2 with brookite particle size and bridged—Nitro surface species. Catal. Commun. 2011, 17, 1–7. [Google Scholar] [CrossRef]
- Dolat, D.; Mozia, S.; Ohtani, B.; Morawski, A.W. Nitrogen, iron-single modified (N-TiO2, Fe-TiO2) and co-modified (Fe, N-TiO2) rutile titanium dioxide as visible-light active photocatalysts. Chem. Eng. J. 2013, 225, 358–364. [Google Scholar] [CrossRef]
- Veréb, G.; Manczinger, L.; Bozsó, G.; Sienkiewicz, A.; Forró, L.; Mogyorósi, K.; Hernádi, K.; Dombi, A. Comparison of the photocatalytic efficiencies of bare and doped rutile and anatase TiO2 photocatalysts under visible light for phenol degradation and E. coli inactivation. Appl. Catal. B 2013, 129, 566–574. [Google Scholar] [CrossRef]
- Emeline, A.V.; Zhang, X.; Jin, M.; Murakami, T.; Fujishima, A. Spectral dependences of the activity and selectivity of N-doped TiO2 in photodegradation of phenols. J. Photochem. Photobiol. A 2009, 207, 13–19. [Google Scholar] [CrossRef]
- Wang, Z.; Cai, W.; Hong, X.; Zhao, X.; Xu, F.; Cai, C. Photocatalytic degradation of phenol in aqueous nitrogen-doped TiO2 suspensions with various light sources. Appl. Catal. B 2005, 57, 223–231. [Google Scholar] [CrossRef]
- Antonopoulou, M.; Giannakas, A.; Bairamis, F.; Papadaki, M.; Konstantinou, I. Degradation of organophosphorus flame retardant tris (1-chloro-2-propyl) phosphate (TCPP) by visible light N, S-codoped TiO2 photocatalysts. Chem. Eng. J. 2016, 318, 231–239. [Google Scholar] [CrossRef]
- Song, S.; Tu, J.J.; He, Z.Q.; Hong, F.Y.; Liu, W.P.; Chen, J.M. Visible light-driven iodine-doped titanium dioxide nanotubes prepared by hydrothermal process and post-calcination. Appl. Catal. A 2010, 378, 169–174. [Google Scholar] [CrossRef]
- He, Z.Q.; Wang, C.; Wang, H.Y.; Hong, F.Y.; Xu, X.H.; Chen, J.M.; Song, S. Increasing the catalytic activities of iodine doped titanium dioxide by modifying with tin dioxide for the photodegradation of 2-chlorophenol under visible light irradiation. J. Hazard. Mater. 2011, 189, 595–602. [Google Scholar] [CrossRef]
- He, Z.Q.; Zhan, L.Y.; Hong, F.Y.; Song, S.; Lin, Z.Y.; Chen, J.M.; Jin, M.T. A visible light-responsive iodine-doped titanium dioxide nanosphere. J. Environ. Sci.-China 2011, 23, 166–170. [Google Scholar] [CrossRef]
- Veréb, G.; Manczinger, L.; Oszkó, A.; Sienkiewicz, A.; Forró, L.; Mogyorósi, K.; Dombi, A.; Hernádia, K. Highly efficient bacteria inactivation and phenol degradation by visible light irradiated iodine doped TiO2. Appl. Catal. B 2013, 129, 194–201. [Google Scholar]
- Guo, S.; Han, S.; Haifeng, M.; Zeng, C.; Sun, Y.; Chi, B.; Pu, J.; Li, J. Synthesis of phosphorus-doped titania with mesoporous structure and excellent photocatalytic activity. Mater. Res. Bull. 2013, 48, 3032–3036. [Google Scholar] [CrossRef]
- Thind, S.S.; Wu, G.; Chen, A. Synthesis of mesoporous nitrogen–tungsten co-doped TiO2 photocatalysts with high visible light activity. Appl. Catal. B 2012, 111–112, 38–45. [Google Scholar] [CrossRef]
- Balazs, N.; Sranko, D.F.; Dombi, A.; Sipos, P.; Mogyorosi, K. The effect of particle shape on the activity of nanocrystalline TiO(2) photocatalysts in phenol decomposition. Part 2: The key synthesis parameters influencing the particle shape and activity. Appl. Catal. B 2010, 96, 569–576. [Google Scholar] [CrossRef]
- Veréb, G.; Ambrus, Z.; Pap, Z.; Kmetykó, Á.; Dombi, A.; Danciu, V.; Cheesman, A.; Mogyorósi, K. Comparative study on UV and visible light sensitive bare and doped titanium dioxide photocatalysts for the decomposition of environmental pollutants in water. Appl. Catal. A Gen. 2012, 417–418, 26–36. [Google Scholar] [CrossRef]
- Hatchard, C.G.; Parker, C.A. A new sensitive chemical actinometer-II. Potassium ferrioxalate as a standard chemical actinometer. Proc. R. Soc. Lond. Ser. A Math. Phys. Sci. 1956, A235, 518–536. [Google Scholar]
- Fischer, E. Ferri-oxalate actinometry. Newsletters 1984, 21, 33–34. [Google Scholar]
- Montalti, M.; Credi, A.; Prodi, L.; Gandolfi, M.T. Handbook of Photochemistry, 3rd ed.; Taylor & Francis Group: Abingdon, UK, 2006. [Google Scholar]





| Irradiation Type | Initial Degradation Rate of Phenol (mol·dm−3·s−1) | |||||
|---|---|---|---|---|---|---|
| TiO2-AA | TiO2-FH | TiO2-P25 | TiO2-AR | TiO2-N | TiO2-VLP7000 | |
| UV | 5.8·10−8 | 1.3·10−7 | 1.2·10−7 | 3.8·10−8 | 1.6·10−8 | 2.3·10−8 |
| Violet | 1.8·10−9 | 5.6·10−9 | 7.0·10−9 | 1.3·10−8 | 1.0·10−8 | 1.8·10−8 |
| Blue | - | 1.3·10−9 | 3.9·10−9 | 5.7·10−10 | 5.9·10−9 | 2.6·10−8 |
| Green | - | - | - | - | 8.9·10−10 | 1.8·10−8 |
| Yellow | - | - | - | - | - | 3.6·10−9 |
| Red | - | - | - | - | - | 5.8·10−9 |
| Irradiation | UV | Violet | Blue (Using 0.02 M Fe-Oxalate Solution) | Blue (Using 0.15 M Fe-Oxalate Solution) |
|---|---|---|---|---|
| Incident photon flux (mol·dm−3·s−1) | 9.23·10−6 | 7.75·10−6 | 4.25·10−5 | 5.16·10−5 |
| Irradiation Type | Ratio of Photon Flux Belonging to Actual Irradiation and Photon Flux of Blue LED (Measured by PPF Meter) | Calculated Incident Photon Flux (mol·dm−3·s−1) |
|---|---|---|
| blue LED | 1.00 | 5.16·10−5 |
| green LED | 0.69 | 3.56·10−5 |
| yellow LED | 0.21 | 1.08·10−5 |
| red LED | 1.14 | 5.88·10−5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Veréb, G.; Gyulavári, T.; Virág, O.; Alapi, T.; Hernadi, K.; Pap, Z. Wavelength Dependence of the Photocatalytic Performance of Pure and Doped TiO2 Photocatalysts—A Reflection on the Importance of UV Excitability. Catalysts 2022, 12, 1492. https://doi.org/10.3390/catal12121492
Veréb G, Gyulavári T, Virág O, Alapi T, Hernadi K, Pap Z. Wavelength Dependence of the Photocatalytic Performance of Pure and Doped TiO2 Photocatalysts—A Reflection on the Importance of UV Excitability. Catalysts. 2022; 12(12):1492. https://doi.org/10.3390/catal12121492
Chicago/Turabian StyleVeréb, Gábor, Tamás Gyulavári, Orsolya Virág, Tünde Alapi, Klara Hernadi, and Zsolt Pap. 2022. "Wavelength Dependence of the Photocatalytic Performance of Pure and Doped TiO2 Photocatalysts—A Reflection on the Importance of UV Excitability" Catalysts 12, no. 12: 1492. https://doi.org/10.3390/catal12121492