Fabrication of TaON/CdS Heterostructures for Enhanced Photocatalytic Hydrogen Evolution under Visible Light Irradiation
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. The Preparation of TaON Particles
2.3. The Preparation of TaON/CdS Nanocomposites
2.4. General Characterization
2.5. Photoelectrochemical Testing
2.6. Photocatalytic Hydrogen Production
3. Results and Discussion
3.1. Catalyst Characterization
3.2. Photocatalytic Performance Tests
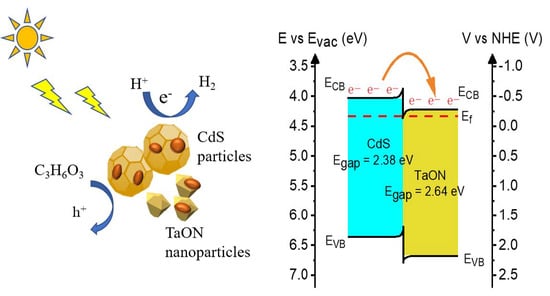
3.3. Photocatalytic Mechanism Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hou, J.G.; Wang, Z.; Kan, W.B.; Jiao, S.Q.; Zhu, H.M.; Kumar, R.V. Efficient visible-light-driven photocatalytic hydrogen production using CdS@TaON core-shell composites coupled with graphene oxide nanosheets. J. Mater. Chem. 2012, 22, 7291–7299. [Google Scholar] [CrossRef]
- Zou, Y.Y.; Guo, C.Y.; Cao, X.L.; Zhang, L.G.; Chen, T.X.; Guo, C.; Wang, J.D. Synthesis of CdS/CoP hollow nanocages with improved photocatalytic water splitting performance for hydrogen evolution. J. Environ. Chem. Eng. 2021, 9, 106270. [Google Scholar] [CrossRef]
- Lei, Y.; Wu, X.; Li, S.; Huang, J.; Ng, K.H.; Lai, Y. Noble-metal-free metallic MoC combined with CdS for enhanced visible-light-driven photocatalytic hydrogen evolution. J. Clean. Prod. 2021, 322, 129018. [Google Scholar] [CrossRef]
- Sun, G.; Xiao, B.; Shi, J.-W.; Mao, S.; He, C.; Ma, D.; Cheng, Y. Hydrogen spillover effect induced by ascorbic acid in CdS/NiO core-shell p-n heterojunction for significantly enhanced photocatalytic H2 evolution. J. Colloid Interface Sci. 2021, 596, 215–224. [Google Scholar] [CrossRef]
- El-Hakam, S.A.; Alshorifi, F.T.; Salama, R.S.; Gamal, S.; El-Yazeed, W.S.A.; Ibrahim, A.A.; Ahmed, A.I. Application of nanostructured mesoporous silica/ bismuth vanadate composite catalysts for the degradation of methylene blue and brilliant green. J. Mater. Res. Technol. 2022, 18, 1963–1976. [Google Scholar] [CrossRef]
- Alshorifi, F.T.; Alswat, A.A.; Mannaa, M.A.; Alotaibi, M.T.; El-Bahy, S.M.; Salama, R.S. Facile and Green Synthesis of Silver Quantum Dots Immobilized onto a Polymeric CTS–PEO Blend for the Photocatalytic Degradation of p-Nitrophenol. ACS Omega 2021, 6, 30432–30441. [Google Scholar] [CrossRef]
- Alshorifi, F.T.; Ali, S.L.; Salama, R.S. Promotional Synergistic Effect of Cs-Au NPs on the Performance of Cs-Au/MgFe2O4 Catalysts in Catalysis 3,4-Dihydropyrimidin-2(1H)-Ones and Degradation of RhB Dye. J. Inorg. Organomet. Polym. Mater. 2022, 32, 1–12. [Google Scholar] [CrossRef]
- Gai, Q.; Zheng, X.; Liu, W.; Dong, Q.; Wang, Y.; Gao, R.; Ren, S. 2D-2D heterostructured CdS–CoP photocatalysts for efficient H2 evolution under visible light irradiation. Int. J. Hydrog. Energy 2019, 44, 27412–27420. [Google Scholar] [CrossRef]
- Zhong, W.W.; Tu, W.G.; Feng, S.S.; Xu, A.J. Photocatalytic H2 evolution on CdS nanoparticles by loading FeSe nanorods as co-catalyst under visible light irradiation. J. Alloy. Compd. 2019, 772, 669–674. [Google Scholar] [CrossRef]
- Wu, T.F.; Huang, J.F.; Cheng, G.; Pang, Y.W. Enhanced photocatalytic hydrogen evolution based on ternary noble-metal-free Co3O4/CdS/g-C3N4 composite. Mater. Lett. 2021, 292, 129274. [Google Scholar] [CrossRef]
- An, L.; Han, X.; Li, Y.G.; Hou, C.Y.; Wang, H.Z.; Zhang, Q.H. ZnS-CdS-TaON nanocomposites with enhanced stability and photocatalytic hydrogen evolution activity. J. Sol-Gel Sci. Technol. 2019, 91, 82–91. [Google Scholar] [CrossRef]
- Zhu, R.S.; Yang, R.J.; Hu, L.J.; Chen, B.Y. Preparation of Z-Scheme system of CdS-RGO-BiVO4 and its activity for hydrogen production. Int. J. Hydrog. Energy 2019, 44, 25119–25128. [Google Scholar] [CrossRef]
- Yuan, M.; Zhou, W.H.; Kou, D.X.; Zhou, Z.J.; Meng, Y.N.; Wu, S.X. Cu2ZnSnS4 decorated CdS nanorods for enhanced visible-light-driven photocatalytic hydrogen production. Int. J. Hydrog. Energy 2018, 43, 20408–20416. [Google Scholar] [CrossRef]
- Xu, Y.; Yan, A.; Jiang, L.; Huang, F.; Hu, D.; Duan, G.; Zheng, F. MoS2/HCSs/ZnIn2S4 nanocomposites with enhanced charge transport and photocatalytic hydrogen evolution performance. J. Alloy. Compd. 2022, 895, 162504. [Google Scholar] [CrossRef]
- Kumar, D.P.; Seo, S.; Rangappa, A.P.; Kim, S.; Joshi Reddy, K.A.; Gopannagari, M.; Bhavani, P.; Reddy, D.A.; Kim, T.K. Ultrathin layered Zn-doped MoS2 nanosheets deposited onto CdS nanorods for spectacular photocatalytic hydrogen evolution. J. Alloy. Compd. 2022, 905, 164193. [Google Scholar] [CrossRef]
- Wei, T.T.; Jin, Z.B.; Li, F.Y.; Yan, D.D.; Xu, L. Efficient visible-light-driven photocatalytic hydrogen production over a direct Z-scheme system of TaON/Cd0.5Zn0.5S with a NiS cocatalyst. Photochem. Photobiol. Sci. 2020, 19, 80–87. [Google Scholar] [CrossRef]
- Zhu, Y.; Liu, Y.; Ai, Q.; Gao, G.; Yuan, L.; Fang, Q.; Tian, X.; Zhang, X.; Egap, E.; Ajayan, P.M. In Situ Synthesis of Lead-Free Halide Perovskite–COF Nanocomposites as Photocatalysts for Photoinduced Polymerization in Both Organic and Aqueous Phases. ACS Mater. Lett. 2022, 4, 464–471. [Google Scholar] [CrossRef]
- Yin, X.-L.; Liu, J.; Jiang, W.-J.; Zhang, X.; Hu, J.-S.; Wan, L.-J. Urchin-like Au@CdS/WO3 micro/nano heterostructure as a visible-light driven photocatalyst for efficient hydrogen generation. Chem. Commun. 2015, 51, 13842–13845. [Google Scholar] [CrossRef] [PubMed]
- Hao, X.; Hu, Z.; Xiang, D.; Jin, Z. Construction of CdS@Cu2-xS core−shell p-n heterojunction with enhanced charge separation for wide spectrum photocatalytic H2 evolution. Mol. Catal. 2022, 528, 112417. [Google Scholar] [CrossRef]
- Liu, S.; Xiao, W.; Jin, C.; Xia, S.; Wang, W.; Jiang, X.; Li, L.; Wang, S.; Chen, C. MOFs derived CdS/CdO heterojunction photoanode for high-efficient water splitting. Appl. Surf. Sci. 2022, 605, 154697. [Google Scholar] [CrossRef]
- Liu, C.; Ma, J.; Zhang, F.-J.; Wang, Y.-R.; Kong, C. Facile formation of Mo-vacancy defective MoS2/CdS nanoparticles enhanced efficient hydrogen production. Colloids Surf. A: Physicochem. Eng. Asp. 2022, 643, 128743. [Google Scholar] [CrossRef]
- Han, B.; Liu, S.Q.; Zhang, N.; Xu, Y.J.; Tang, Z.R. One-dimensional CdS@MoS2 core-shell nanowires for boosted photocatalytic hydrogen evolution under visible light. Appl. Catal. B Environ. 2017, 202, 298–304. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, Y.; Chen, G.; Guo, T.; Wang, L.; Huang, X.; Zeng, W. Loading cobalt phosphate on TaON surface as efficient noble-metal-free co-catalyst for enhanced photocatalytic water oxidation performance. Mater. Lett. 2015, 148, 155–158. [Google Scholar] [CrossRef]
- Hou, J.G.; Cheng, H.J.; Yang, C.; Takeda, O.; Zhu, H.M. Hierarchical carbon quantum dots/hydrogenated-gamma-TaON heterojunctions for broad spectrum photocatalytic performance. Nano Energy 2015, 18, 143–153. [Google Scholar] [CrossRef]
- Chen, S.S.; Qi, Y.; Hisatomi, T.; Ding, Q.; Asai, T.; Li, Z.; Ma, S.S.K.; Zhang, F.X.; Domen, K.; Li, C. Efficient Visible-Light-Driven Z-Scheme Overall Water Splitting Using a MgTa2O6-xNy/TaON Heterostructure Photocatalyst for H-2 Evolution. Angew. Chem. Int. Ed. 2015, 54, 8498–8501. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Chu, M.C.; Gao, L.; Mao, L.Q.; Yuan, J.; Shangguan, W.F. Ni(OH)2 loaded on TaON for enhancing photocatalytic water splitting activity under visible light irradiation. Appl. Surf. Sci. 2015, 324, 432–437. [Google Scholar] [CrossRef]
- Yan, J.S.; Hu, L.Q.; Cui, L.K.; Shen, Q.Q.; Liu, X.G.; Jia, H.S.; Xue, J.B. Synthesis of disorder-order TaON homojunction for photocatalytic hydrogen generation under visible light. J. Mater. Sci. 2021, 56, 9791–9806. [Google Scholar] [CrossRef]
- Hou, J.; Yang, C.; Wang, Z.; Ji, Q.; Li, Y.; Huang, G.; Jiao, S.; Zhu, H. Three-dimensional Z-scheme AgCl/Ag/gamma-TaON heterostructural hollow spheres for enhanced visible-light photocatalytic performance. Appl. Catal. B Environ. 2013, 142, 579–589. [Google Scholar] [CrossRef]
- Hai, X.; Chang, K.; Pang, H.; Li, M.; Li, P.; Liu, H.M.; Shi, L.; Ye, J.H. Engineering the Edges of MoS2 (WS2) Crystals for Direct Exfoliation into Monolayers in Polar Micromolecular Solvents. J. Am. Chem. Soc. 2016, 138, 14962–14969. [Google Scholar] [CrossRef]
- Pei, L.; Yuan, Y.J.; Zhong, J.S.; Li, T.Z.; Yang, T.; Yan, S.C.; Ji, Z.G.; Zou, Z.G. Ta3N5 nanorods encapsulated into 3D hydrangea-like MoS2 for enhanced photocatalytic hydrogen evolution under visible light irradiation. Dalton Trans. 2019, 48, 13176–13183. [Google Scholar] [CrossRef]
- Yin, X.-L.; Li, L.-L.; Jiang, W.-J.; Zhang, Y.; Zhang, X.; Wan, L.-J.; Hu, J.-S. MoS2/CdS Nanosheets-on-Nanorod Heterostructure for Highly Efficient Photocatalytic H2 Generation under Visible Light Irradiation. ACS Appl. Mater. Interfaces 2016, 8, 15258–15266. [Google Scholar] [CrossRef] [PubMed]
- Di, T.; Zhang, L.; Cheng, B.; Yu, J.; Fan, J. CdS nanosheets decorated with Ni@graphene core-shell cocatalyst for superior photocatalytic H2 production. J. Mater. Sci. Technol. 2020, 56, 170–178. [Google Scholar] [CrossRef]
- Yin, X.-L.; Li, L.-L.; Li, D.-C.; Li, Z.-J.; Wang, Y.-X.; Kong, X.-J.; Zhao, J.-S.; Jiang, J.-H.; Qian, J.-C.; Pang, D.H. Noble-metal-free CdS@MoS2 core-shell nanoheterostructures for efficient and stabilized visible-light-driven H2 generation. Int. J. Hydrog. Energy 2019, 44, 16657–16666. [Google Scholar] [CrossRef]
- Tang, S.; Xia, Y.; Fan, J.; Cheng, B.; Yu, J.; Ho, W. Enhanced photocatalytic H2 production performance of CdS hollow spheres using C and Pt as bi-cocatalysts. Chin. J. Catal. 2021, 42, 743–752. [Google Scholar] [CrossRef]
- Maeda, K.; Terashima, H.; Kase, K.; Domen, K. Nanoparticulate precursor route to fine particles of TaON and ZrO2–TaON solid solution and their photocatalytic activity for hydrogen evolution under visible light. Appl. Catal. A Gen. 2009, 357, 206–212. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Zeng, C.M.; Ai, L.H.; Jiang, J. Boosting charge transfer and hydrogen evolution performance of CdS nanocrystals hybridized with MoS2 nanosheets under visible light irradiation. Appl. Surf. Sci. 2019, 484, 692–700. [Google Scholar] [CrossRef]
- Zubair, M.; Vanhaecke, E.M.M.; Svenum, I.H.; Ronning, M.; Yang, J. Core-shell particles of C-doped CdS and graphene: A noble metal-free approach for efficient photocatalytic H2 generation. Green Energy Environ. 2020, 5, 461–472. [Google Scholar] [CrossRef]
- Yang, G.W.; Chen, T.; Xing, C.W.; Tian, Z.C.; Hu, Y.J.; Yu, G.Y.; Li, X.Y. Construction of multi-scale 1D/2D CdS/ZnS(en)(0.5) nanorod/nanosheet heterojunction to boost photocatalytic hydrogen generation performance. Appl. Surf. Sci. 2022, 578, 152033. [Google Scholar] [CrossRef]
- Wang, J.H.; Zhu, Q.; Liao, Y.W.; Fu, H.Q.; Chang, J.M.; Zhang, Y.L.; Kan, T.T.; Gao, H.J.; Huang, W.C. Efficient enhancement of photocatalytic hydrogen evolution of CdS nanorods by Nano-CuO. J. Alloy. Compd. 2021, 883, 160832. [Google Scholar] [CrossRef]
- Zhang, J.; Qiao, S.Z.; Qi, L.F.; Yu, J.G. Fabrication of NiS modified CdS nanorod p-n junction photocatalysts with enhanced visible-light photocatalytic H2 production activity. Phys. Chem. Chem. Phys. 2013, 15, 12088–12094. [Google Scholar] [CrossRef] [Green Version]
- Sun, H.; Chen, J.; Liu, S.; Agrawal, D.K.; Zhao, Y.; Wang, D.; Mao, Z. Photocatalytic H2 evolution of porous silicon derived from magnesiothermic reduction of mesoporous SiO2. Int. J. Hydrog. Energy 2019, 44, 7216–7221. [Google Scholar] [CrossRef]
- Wang, W.; Fang, J. Mesoporous SiO2-derived g-C3N4@CdS core-shell heteronanostructure for efficient and stable photocatalytic H2 production. Ceram. Int. 2020, 46, 2384–2391. [Google Scholar] [CrossRef]
- Wang, X.J.; Tian, X.; Sun, Y.J.; Zhu, J.Y.; Li, F.T.; Mu, H.Y.; Zhao, J. Enhanced Schottky effect of a 2D-2D CoP/g-C3N4 interface for boosting photocatalytic H2 evolution. Nanoscale 2018, 10, 12315–12321. [Google Scholar] [CrossRef] [PubMed]










| Photocatalysts | Light Source | Sacrificial Reagent | Activity (mmol g−1 h−1) | AQY (420 nm) | References |
|---|---|---|---|---|---|
| TaON /CdS hybrids | λ > 420 nm | 10% lactic acid | 19.29 | 18.23% | This work |
| MoS2 /CdS nanocrystals | λ > 420 nm | 10% TEOA | 1.15 | 0.66% | [36] |
| C-doped CdS nanoparticles @Graphene | AM 1.5G | 0.125 M Na2S 0.15 M Na2SO3 | 3.12 | 11.7% | [37] |
| CdS nanorods /ZnS nanosheets | λ > 420 nm | 0.025 M NaH2PO2 | 9.44 | / | [38] |
| CuO /CdS nanorods | λ > 420 nm | 8% lactic acid | 3.317 | 6.3% | [39] |
| NiS /CdS nanorods | λ > 420 nm | 0.35 M Na2S 0.25 M Na2SO3 | 1.131 | 6.1% | [40] |
| mesoporous SiO2 | λ > 420 nm | 10% TEOA | 0.607 | / | [41] |
| SiO2-derived g-C3N4@CdS | λ > 420 nm | 10% TEOA | 2.73 | / | [42] |
| Samples | Rct (Ω) | Rs (Ω) | CPE (μF) |
|---|---|---|---|
| CdS | 6.02 × 105 | 6.65 | 121.4 |
| TaON | 8.63 × 105 | 8.52 | 211.3 |
| TC2 | 4.28 × 105 | 7.59 | 124.2 |
| TC4 | 1.71 × 105 | 6.06 | 122.3 |
| TC6 | 3.35 × 105 | 7.62 | 125.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, F.; Fu, H.; Yang, X.; Xiong, S.; An, X. Fabrication of TaON/CdS Heterostructures for Enhanced Photocatalytic Hydrogen Evolution under Visible Light Irradiation. Catalysts 2022, 12, 1110. https://doi.org/10.3390/catal12101110
Chen F, Fu H, Yang X, Xiong S, An X. Fabrication of TaON/CdS Heterostructures for Enhanced Photocatalytic Hydrogen Evolution under Visible Light Irradiation. Catalysts. 2022; 12(10):1110. https://doi.org/10.3390/catal12101110
Chicago/Turabian StyleChen, Fu, Haitao Fu, Xiaohong Yang, Shixian Xiong, and Xizhong An. 2022. "Fabrication of TaON/CdS Heterostructures for Enhanced Photocatalytic Hydrogen Evolution under Visible Light Irradiation" Catalysts 12, no. 10: 1110. https://doi.org/10.3390/catal12101110