Waste Biomass Selective and Sustainable Photooxidation to High-Added-Value Products: A Review
Abstract
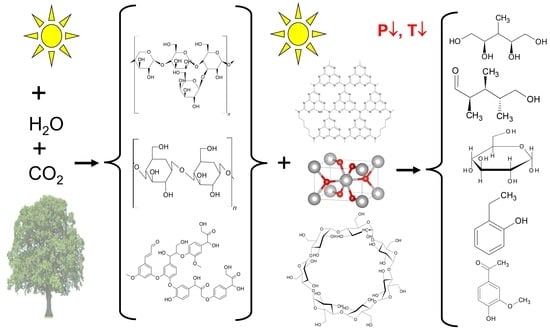
:1. Introduction
2. Materials and Methods
3. Biomass
4. Heterogeneous Photocatalysis
5. Photocatalysts for Biomass Conversion
5.1. Titanium Dioxide TiO2
5.2. Carbon Nitride (g-C3N4)
5.3. Doping with Noble Metals
5.4. Heterojunctions
5.5. Photocatalysts Modified by Macromolecules
6. Lignocellulosic Biomass Conversion Using Photocatalysts
6.1. Cellulose Conversion
6.2. Lignin Conversion
7. Perspectives and Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, S.H.; Liu, S.; Colmenares, J.C.; Xu, Y.J. A Sustainable Approach for Lignin Valorization by Heterogeneous Photocatalysis. Green Chem. 2016, 18, 594–607. [Google Scholar] [CrossRef]
- Granone, L.I.; Sieland, F.; Zheng, N.; Dillert, R.; Bahnemann, D.W. Photocatalytic Conversion of Biomass into Valuable Products: A Meaningful Approach? Green Chem. 2018, 20, 1169–1192. [Google Scholar] [CrossRef]
- Gallezot, P. Conversion of Biomass to Selected Chemical Products. Chem. Soc. Rev. 2012, 41, 1538–1558. [Google Scholar] [CrossRef]
- Sheldon, R.A. Green and Sustainable Manufacture of Chemicals from Biomass: State of the Art. Green Chem. 2014, 16, 950–963. [Google Scholar] [CrossRef]
- Colmenares, J.C.; Luque, R. Heterogeneous Photocatalytic Nanomaterials: Prospects and Challenges in Selective Trans-formations of Biomass-Derived Compounds. Chem. Soc. Rev. 2014, 43, 765–778. [Google Scholar] [CrossRef]
- Jing, Y.; Guo, Y.; Xia, Q.; Liu, X.; Wang, Y. Catalytic Production of Value-Added Chemicals and Liquid Fuels from Lignocellosic Biomass. Chem 2019, 5, 2520–2546. [Google Scholar] [CrossRef]
- Lan, J.; Lin, J.; Chen, Z.; Yin, G. Transformation of 5-Hydroxymethylfurfural (HMF) to Maleic Anhydride by Aerobic Oxidation with Heteropolyacid Catalysts. ACS Catal. 2015, 5, 2035–2041. [Google Scholar] [CrossRef]
- Clarizia, L.; Apuzzo, J.; Di Somma, I.; Marotta, R.; Andreozzi, R. Selective Photo-Oxidation of Ethanol to Acetaldehyde and Acetic Acid in Water in Presence of TiO2 and Cupric Ions under U.V.–Simulated Solar Radiation. Chem. Eng. J. 2019, 361, 1524–1534. [Google Scholar] [CrossRef]
- Payormhorm, J.; Chuangchote, S.; Kiatkittipong, K.; Chiarakorn, S.; Laosiripojana, N. Xylitol and Gluconic Acid Productions via Photocatalytic-Glucose Conversion Using TiO2 Fabricated by Surfactant-Assisted Techniques: Effects of Structural and Textural Properties. Mater. Chem. Phys. 2017, 196, 29–36. [Google Scholar] [CrossRef]
- Wu, X.; Fan, X.; Xie, S.; Lin, J.; Cheng, J.; Zhang, Q.; Chen, L.; Wang, Y. Solar Energy-Driven Lignin-First Approach to Full Utilization of Lignocellulosic Biomass under Mild Conditions. Nat. Catal. 2018, 1, 772–780. [Google Scholar] [CrossRef]
- Liu, X.; Duan, X.; Wei, W.; Wang, S.; Ni, B.J. Photocatalytic Conversion of Lignocellulosic Biomass to Valuable Products. Green Chem. 2019, 21, 4266–4289. [Google Scholar] [CrossRef]
- Singhvi, M.S.; Gokhale, D.V. Lignocellulosic Biomass: Hurdles and Challenges in Its Valorization. Appl. Microbiol. Biotechnol. 2019, 103, 9305–9320. [Google Scholar] [CrossRef]
- Scott, E.; Peter, F.; Sanders, J. Biomass in the Manufacture of Industrial Products—the Use of Proteins and Amino Acids. Appl. Microbiol. Biotechnol. 2007, 75, 751–762. [Google Scholar] [CrossRef]
- Girisuta, B.; Heeres, H.J. Levulinic Acid from Biomass: Synthesis and Applications. In Production of Platform Chemicals from Sustainable Resources; Springer: Berlin/Heidelberg, Germany, 2017; pp. 143–169. [Google Scholar] [CrossRef]
- Tang, X.; Zeng, X.; Li, Z.; Hu, L.; Sun, Y.; Liu, S.; Lei, T.; Lin, L. Production of γ-Valerolactone from Lignocellulosic Biomass for Sustainable Fuels and Chemicals Supply. Renew. Sustain. Energy Rev. 2014, 40, 608–620. [Google Scholar] [CrossRef]
- Zhou, H.; Xu, H.; Wang, X.; Liu, Y. Convergent Production of 2,5-Furandicarboxylic Acid from Biomass and CO2. Green Chem. 2019, 21, 2923–2927. [Google Scholar] [CrossRef]
- Verdini, F.; Gaudino, E.C.; Canova, E.; Tabasso, S.; Behbahani, P.J.; Cravotto, G. Lignin as a Natural Carrier for the Efficient Delivery of Bioactive Compounds: From Waste to Health. Molecules 2022, 27, 3598. [Google Scholar] [CrossRef]
- Wang, S.; Dai, G.; Yang, H.; Luo, Z. Lignocellulosic Biomass Pyrolysis Mechanism: A State-of-the-Art Review. Prog. Energy Combust. Sci. 2017, 62, 33–86. [Google Scholar] [CrossRef]
- Chai, L.; Hou, X.; Cui, X.; Li, H.; Zhang, N.; Zhang, H.; Chen, C.; Wang, Y.; Deng, T. 5-Hydroxymethylfurfural Oxidation to Maleic Acid by O2 over Graphene Oxide Supported Vanadium: Solvent Effects and Reaction Mechanism. Chem. Eng. J. 2020, 388, 124187. [Google Scholar] [CrossRef]
- Ahorsu, R.; Medina, F.; Constantí, M. Significance and Challenges of Biomass as a Suitable Feedstock for Bioenergy and Bio-chemical Production: A Review. Energies 2018, 11, 3366. [Google Scholar] [CrossRef] [Green Version]
- Bellardita, M.; Loddo, V.; Palmisano, L. Formation of High Added Value Chemicals by Photocatalytic Treatment of Biomass. Mini-Rev. Org. Chem. 2020, 17, 884–901. [Google Scholar] [CrossRef]
- Fonseca-Cervantes, O.R.; Pérez-Larios, A.; Romero Arellano, V.H.; Sulbaran-Rangel, B.; González, C.A.G. Effects in Band Gap for Photocatalysis in TiO2 Support by Adding Gold and Ruthenium. Processes 2020, 8, 1032. [Google Scholar] [CrossRef]
- Wu, X.; Chang, Y.; Lin, S. Titanium Radical Redox Catalysis: Recent Innovations in Catalysts, Reactions, and Modes of Activation. Chem 2022, 8, 1805–1821. [Google Scholar] [CrossRef]
- Patnaik, S.; Sahoo, D.P.; Parida, K.M. Bimetallic Co-Effect of Au-Pd Alloyed Nanoparticles on Mesoporous Silica Modified g-C3N4 for Single and Simultaneous Photocatalytic Oxidation of Phenol and Reduction of Hexavalent Chromium. J. Colloid Interface Sci. 2020, 560, 519–535. [Google Scholar] [CrossRef]
- Chen, K.; Shen, T.; Lu, Y.; Hu, Y.; Wang, J.; Zhang, J.; Wang, D. Engineering Titanium Oxide-Based Support for Electrocatalysis. J. Energy Chem. 2022, 67, 168–183. [Google Scholar] [CrossRef]
- Wu, X.; Li, J.; Xie, S.; Duan, P.; Zhang, H.; Feng, J.; Zhang, Q.; Cheng, J.; Wang, Y. Selectivity Control in Photocatalytic Valorization of Biomass-Derived Platform Compounds by Surface Engineering of Titanium Oxide. Chem 2020, 6, 3038–3053. [Google Scholar] [CrossRef]
- Zhao, H.; Li, C.F.; Liu, L.Y.; Palma, B.; Hu, Z.Y.; Renneckar, S.; Larter, S.; Li, Y.; Kibria, M.G.; Hu, J.; et al. N-p Heterojunction of TiO2-NiO Core-Shell Structure for Efficient Hydrogen Generation and Lignin Photoreforming. J. Colloid Interface Sci. 2021, 585, 694–704. [Google Scholar] [CrossRef]
- Segovia-Guzmán, M.O.; Román-Aguirre, M.; Verde-Gomez, J.Y.; Collins-Martínez, V.H.; Zaragoza-Galán, G.; Ramos-Sánchez, V.H. Green Cu2O/TiO2 Heterojunction for Glycerol Photoreforming. Catal. Today 2020, 349, 88–97. [Google Scholar] [CrossRef]
- Li, Y.; Yin, Q.; Zeng, Y.; Liu, Z. Hollow Spherical Biomass Derived-Carbon Dotted with SnS2/g-C3N4 Z-Scheme Heterojunction for Efficient CO2 Photoreduction into CO. Chem. Eng. J. 2022, 438, 135652. [Google Scholar] [CrossRef]
- Yang, Q.; Wang, T.; Han, F.; Zheng, Z.; Xing, B.; Li, B. Bimetal-Modified g-C3N4 Photocatalyst for Promoting Hydrogen Pro-duction Coupled with Selective Oxidation of Biomass Derivative. J. Alloys Compd. 2022, 897, 163177. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, Y.; Cheng, L.; Ismael, M.; Feng, Z.; Wu, Y. Novel Application of G-C3N4/NaNbO3 Composite for Photocatalytic Selective Oxidation of Biomass-Derived HMF to FFCA under Visible Light Irradiation. Adv. Powder Technol. 2020, 31, 1148–1159. [Google Scholar] [CrossRef]
- Zhang, H.; Feng, Z.; Zhu, Y.; Wu, Y.; Wu, T. Photocatalytic Selective Oxidation of Biomass-Derived 5-Hydroxymethylfurfural to 2,5-Diformylfuran on WO3/g-C3N4 Composite under Irradiation of Visible Light. J. Photochem. Photobiol. A Chem. 2019, 371, 1–9. [Google Scholar] [CrossRef]
- Patnaik, S.; Sahoo, D.P.; Parida, K. Recent Advances in Anion Doped G-C3N4 Photocatalysts: A Review. Carbon N. Y. 2021, 172, 682–711. [Google Scholar] [CrossRef]
- Cai, B.; Zhang, Y.; Feng, J.; Huang, C.; Ma, T.; Pan, H. Highly Efficient G-C3N4 Supported Ruthenium Catalysts for the Catalytic Transfer Hydrogenation of Levulinic Acid to Liquid Fuel γ-Valerolactone. Renew. Energy 2021, 177, 652–662. [Google Scholar] [CrossRef]
- Payormhorm, J.; Idem, R. Synthesis of C-Doped TiO2 by Sol-Microwave Method for Photocatalytic Conversion of Glycerol to Value-Added Chemicals under Visible Light. Appl. Catal. A Gen. 2020, 590, 117362. [Google Scholar] [CrossRef]
- Yoon, T.P.; Ischay, M.A.; Du, J. Visible Light Photocatalysis as a Greener Approach to Photochemical Synthesis. Nat. Chem. 2010, 2, 527–532. [Google Scholar] [CrossRef]
- Wu, X.; Luo, N.; Xie, S.; Zhang, H.; Zhang, Q.; Wang, F.; Wang, Y. Photocatalytic Transformations of Lignocellulosic Biomass into Chemicals. Chem. Soc. Rev. 2020, 49, 6198–6223. [Google Scholar] [CrossRef]
- Yan, K.; Li, H. State of the Art and Perspectives in Catalytic Conversion Mechanism of Biomass to Bio-aromatics. Energy Fuels 2021, 35, 45–62. [Google Scholar] [CrossRef]
- Heng, Z.W.; Chong, W.C.; Pang, Y.L.; Koo, C.H. An Overview of the Recent Advances of Carbon Quantum Dots/Metal Oxides in the Application of Heterogeneous Photocatalysis in Photodegradation of Pollutants towards Visible-Light and Solar Energy Exploitation. J. Environ. Chem. Eng. 2021, 9, 105199. [Google Scholar] [CrossRef]
- Lang, X.; Chen, X.; Zhao, J. Heterogeneous Visible Light Photocatalysis for Selective Organic Transformations. Chem. Soc. Rev. 2014, 43, 473–486. [Google Scholar] [CrossRef]
- Yousuf, A.; Pirozzi, D.; Sannino, F. Fundamentals of Lignocellulosic Biomass. Lignocellul. Biomass Liq. Biofuels 2020, 1–15. [Google Scholar] [CrossRef]
- Colmenares, J.C.; Magdziarz, A. Room Temperature Versatile Conversion of Biomass-Derived Compounds by Means of Supported TiO2 Photocatalysts. J. Mol. Catal. A Chem. 2013, 366, 156–162. [Google Scholar] [CrossRef]
- Nguyen, J.D.; Matsuura, B.S.; Stephenson, C.R.J. A Photochemical Strategy for Lignin Degradation at Room Temperature. J. Am. Chem. Soc. 2014, 136, 1218–1221. [Google Scholar] [CrossRef]
- Zhang, Y.; Naebe, M. Lignin: A Review on Structure, Properties, and Applications as a Light-Colored UV Absorber. ACS Sustain. Chem. Eng. 2021, 9, 1427–1442. [Google Scholar] [CrossRef]
- Sannigrahi, P.; Pu, Y.; Ragauskas, A. Cellulosic Biorefineries-Unleashing Lignin Opportunities. Curr. Opin. Environ. Sustain. 2010, 2, 383–393. [Google Scholar] [CrossRef]
- Xu, C.; Arancon, R.A.D.; Labidi, J.; Luque, R. Lignin Depolymerisation Strategies: Towards Valuable Chemicals and Fuels. Chem. Soc. Rev. 2014, 43, 7485–7500. [Google Scholar] [CrossRef]
- Onokwai, A.O.; Ajisegiri, E.S.A.; Okokpujie, I.P.; Ibikunle, R.A.; Oki, M.; Dirisu, J.O. Characterization of Lignocellulose Biomass Based on Proximate, Ultimate, Structural Composition, and Thermal Analysis. Mater. Today Proc. 2022, 65, 2156–2162. [Google Scholar] [CrossRef]
- Monir, M.U.; Abd Aziz, A.; Kristanti, R.A.; Yousuf, A. Gasification of Lignocellulosic Biomass to Produce Syngas in a 50 kW Downdraft Reactor. Biomass Bioenerg. 2018, 119, 335–345. [Google Scholar] [CrossRef]
- Lee, C.S.; Conradie, A.V.; Lester, E. Review of Supercritical Water Gasification with Lignocellulosic Real Biomass as the Feedstocks: Process Parameters, Biomass Composition, Catalyst Development, Reactor Design and Its Challenges. Chem. Eng. J. 2021, 415, 128837. [Google Scholar] [CrossRef]
- Cai, J.; Rahman, M.M.; Zhang, S.; Sarker, M.; Zhang, X.; Zhang, Y.; Yu, X.; Fini, E.H. Review on Aging of Bio-Oil from Biomass Pyrolysis and Strategy to Slowing Aging. Energy Fuels 2021, 35, 11665–11692. [Google Scholar] [CrossRef]
- Blanco, E.; Sepulveda, C.; Cruces, K.; García-Fierro, J.L.; Ghampson, I.T.; Escalona, N. Conversion of Guaiacol over Metal Carbides Supported on Activated Carbon Catalysts. Catal. Today 2020, 356, 376–383. [Google Scholar] [CrossRef]
- Kohli, K.; Prajapati, R.; Sharma, B.K. Bio-Based Chemicals from Renewable Biomass for Integrated Biorefineries. Energies 2019, 12, 233. [Google Scholar] [CrossRef]
- Ma, B.; Wang, Y.; Guo, X.; Tong, X.; Liu, C.; Wang, Y.; Guo, X. Photocatalytic Synthesis of 2,5-Diformylfuran from 5-Hydroxymethyfurfural or Fructose over Bimetallic Au-Ru Nanoparticles Supported on Reduced Graphene Oxides. Appl. Catal. A Gen. 2018, 552, 70–76. [Google Scholar] [CrossRef]
- Lozano, F.J.; Lozano, R. Assessing the Potential Sustainability Benefits of Agricultural Residues: Biomass Conversion to Syngas for Energy Generation or to Chemicals Production. J. Clean. Prod. 2018, 172, 4162–4169. [Google Scholar] [CrossRef]
- Wang, M.; Ma, J.; Liu, H.; Luo, N.; Zhao, Z.; Wang, F. Sustainable Productions of Organic Acids and Their Derivatives from Biomass via Selective Oxidative Cleavage of C-C Bond. ACS Catal. 2018, 8, 2129–2165. [Google Scholar] [CrossRef]
- Yoganandham, S.T.; Sathyamoorthy, G.; Renuka, R.R. Emerging Extraction Techniques: Hydrothermal Processing. Sustain. Seaweed Technol. 2020, 191–205. [Google Scholar] [CrossRef]
- Li, X.; Shi, J.L.; Hao, H.; Lang, X. Visible Light-Induced Selective Oxidation of Alcohols with Air by Dye-Sensitized TiO2 Photocatalysis. Appl. Catal. B Environ. 2018, 232, 260–267. [Google Scholar] [CrossRef]
- Marcì, G.; García-López, E.I.; Palmisano, L. Polymeric Carbon Nitride (C3N4) as Heterogeneous Photocatalyst for Selective Oxidation of Alcohols to Aldehydes. Catal. Today 2018, 315, 126–137. [Google Scholar] [CrossRef]
- Chen, L.; Tang, J.; Song, L.N.; Chen, P.; He, J.; Au, C.T.; Yin, S.F. Heterogeneous Photocatalysis for Selective Oxidation of Alcohols and Hydrocarbons. Appl. Catal. B Environ. 2019, 242, 379–388. [Google Scholar] [CrossRef]
- Wan, Y.; Zhang, L.; Chen, Y.; Lin, J.; Hu, W.; Wang, S.; Lin, J.; Wan, S.; Wang, Y. One-Pot Synthesis of Gluconic Acid from Biomass-Derived Levoglucosan Using a Au/Cs2.5H0.5PW12O40 Catalyst. Green Chem. 2019, 21, 6318–6325. [Google Scholar] [CrossRef]
- Fung, C.M.; Tang, J.Y.; Tan, L.L.; Mohamed, A.R.; Chai, S.P. Recent Progress in Two-Dimensional Nanomaterials for Photocatalytic Carbon Dioxide Transformation into Solar Fuels. Mater. Today Sustain. 2020, 9, 100037. [Google Scholar] [CrossRef]
- Du, C.; Wang, X.; Chen, W.; Feng, S.; Wen, J.; Wu, Y.A. CO2 Transformation to Multicarbon Products by Photocatalysis and Electrocatalysis. Mater. Today Adv. 2020, 6, 100071. [Google Scholar] [CrossRef]
- Wang, Q.; Domen, K. Particulate Photocatalysts for Light-Driven Water Splitting: Mechanisms, Challenges, and Design Strategies. Chem. Rev. 2020, 120, 919–985. [Google Scholar] [CrossRef]
- Ibhadon, A.O.; Fitzpatrick, P. Heterogeneous Photocatalysis: Recent Advances and Applications. Catalysts 2013, 3, 189–218. [Google Scholar] [CrossRef]
- Akerdi, A.G.; Bahrami, S.H. Application of Heterogeneous Nano-Semiconductors for Photocatalytic Advanced Oxidation of Organic Compounds: A Review. J. Environ. Chem. Eng. 2019, 7, 103283. [Google Scholar] [CrossRef]
- Augugliaro, V.; Camera-Roda, G.; Loddo, V.; Palmisano, G.; Palmisano, L.; Soria, J.; Yurdakal, S. Heterogeneous Photo-catalysis and Photoelectrocatalysis: From Unselective Abatement of Noxious Species to Selective Production of High-Value Chemicals. J. Phys. Chem. Lett. 2015, 6, 1968–1981. [Google Scholar] [CrossRef]
- Li, Z.; Meng, X. New Insight into Reactive Oxidation Species (ROS) for Bismuth-Based Photocatalysis in Phenol Removal. J. Hazard. Mater. 2020, 399, 122939. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, Z.; Chen, J. Applications of Lignin-Derived Catalysts for Green Synthesis. Green Energy Environ. 2019, 4, 210–244. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, J.; Xing, R.; Wang, Y.; Wang, S.; Wei, D.; Li, J.; Chen, Z.; Lü, J. Preparation of Carbon-Supported CdS Photocatalysts with High Performance of Dye Photodegradation Using Cadmium-Enriched Perilla Frutescens Biomass. Inorg. Chem. Commun. 2019, 109, 107559. [Google Scholar] [CrossRef]
- Liu, X.Q.; Chen, W.J.; Jiang, H. Facile Synthesis of Ag/Ag3PO4/AMB Composite with Improved Photocatalytic Performance. Chem. Eng. J. 2017, 308, 889–896. [Google Scholar] [CrossRef]
- Ren, Z.; Chen, F.; Wen, K.; Lu, J. Enhanced Photocatalytic Activity for Tetracyclines Degradation with Ag Modified G-C3N4 Composite under Visible Light. J. Photochem. Photobiol. A Chem. 2020, 389, 112217. [Google Scholar] [CrossRef]
- Zhao, R.; Sun, X.; Jin, Y.; Han, J.; Wang, L.; Liu, F. Au/Pd/g-C3N4 Nanocomposites for Photocatalytic Degradation of Tetracycline Hydrochloride. J. Mater. Sci. 2019, 54, 5445–5456. [Google Scholar] [CrossRef]
- Asencios, Y.J.O.; Lourenço, V.S.; Carvalho, W.A. Removal of Phenol in Seawater by Heterogeneous Photocatalysis Using Activated Carbon Materials Modified with TiO2. Catal. Today 2022, 388–389, 247–258. [Google Scholar] [CrossRef]
- Khan, A.A.; Tahir, M. Recent Advancements in Engineering Approach towards Design of Photo-Reactors for Selective Photocatalytic CO2 Reduction to Renewable Fuels. J. CO2 Util. 2019, 29, 205–239. [Google Scholar] [CrossRef]
- Kuriki, R.; Yamamoto, M.; Higuchi, K.; Yamamoto, Y.; Akatsuka, M.; Lu, D.; Yagi, S.; Yoshida, T.; Ishitani, O.; Maeda, K. Robust Binding between Carbon Nitride Nanosheets and a Binuclear Ruthenium(II) Complex Enabling Durable, Selective CO2 Reduction under Visible Light in Aqueous Solution. Angew. Chemie Int. Ed. 2017, 56, 4867–4871. [Google Scholar] [CrossRef]
- Ahmed, N.; Morikawa, M.; Izumi, Y. Photocatalytic Conversion of Carbon Dioxide into Methanol Us-ing Optimized Layered Double Hydroxide Catalysts. Catal. Today 2012, 185, 263–269. [Google Scholar] [CrossRef]
- Tasbihi, M.; Schwarze, M.; Edelmannová, M.; Spöri, C.; Strasser, P.; Schomäcker, R. Photocatalytic Reduction of CO2 to Hydrocarbons by Using Photodeposited Pt Nanoparticles on Carbon-Doped Titania. Catal. Today 2019, 328, 8–14. [Google Scholar] [CrossRef]
- Meng, S.; Ye, X.; Zhang, J.; Fu, X.; Chen, S. Effective Use of Photogenerated Electrons and Holes in a System: Photocatalytic Selective Oxidation of Aromatic Alcohols to Aldehydes and Hydrogen Production. J. Catal. 2018, 367, 159–170. [Google Scholar] [CrossRef]
- López-Tenllado, F.J.; Marinas, A.; Urbano, F.J.; Colmenares, J.C.; Hidalgo, M.C.; Marinas, J.M.; Moreno, J.M. Selective Photooxidation of Alcohols as Test Reaction for Photocatalytic Activity. Appl. Catal. B Environ. 2012, 128, 150–158. [Google Scholar] [CrossRef]
- Yuan, B.; Zhang, B.; Wang, Z.; Lu, S.; Li, J.; Liu, Y.; Li, C. Photocatalytic Aerobic Oxidation of Toluene and Its Derivatives to Aldehydes on Pd/Bi2WO6. Chin. J. Catal. 2017, 38, 440–446. [Google Scholar] [CrossRef]
- Thakur, S.; Kaur, K.; Das, N.; Pal, B. Photo-Oxidation Kinetics of Sugars Having Different Molecular Size and Glycosidic Linkages for Their Complete Mineralization to Subunits by Bare/Ag–TiO2 under U.V. Irradiation. J. Taiwan Inst. Chem. Eng. 2017, 80, 488–494. [Google Scholar] [CrossRef]
- Albert, J. Selective Oxidation of Lignocellulosic Biomass to Formic Acid and High-Grade Cellulose Using Tailor-Made Polyoxometalate Catalysts. Faraday Discuss. 2017, 202, 99–109. [Google Scholar] [CrossRef]
- Li, X.; Chen, Y.; Tao, Y.; Shen, L.; Xu, Z.; Bian, Z.; Li, H. Challenges of Photocatalysis and Their Coping Strategies. Chem. Catal. 2022, 2, 1315–1345. [Google Scholar] [CrossRef]
- Jin, C.; Dai, Y.; Wei, W.; Ma, X.; Li, M.; Huang, B. Effects of Single Metal Atom (Pt, Pd, Rh and Ru) Adsorption on the Photocatalytic Properties of Anatase TiO2. Appl. Surf. Sci. 2017, 426, 639–646. [Google Scholar] [CrossRef]
- Wakerley, D.W.; Kuehnel, M.F.; Orchard, K.L.; Ly, K.H.; Rosser, T.E.; Reisner, E. Solar-Driven Re-forming of Lignocellulose to H2 with a CdS/CdOx Photocatalyst. Nat. Energy 2017, 24, 17021. [Google Scholar] [CrossRef]
- Zhao, H.; Li, C.F.; Yu, X.; Zhong, N.; Hu, Z.Y.; Li, Y.; Larter, S.; Kibria, M.G.; Hu, J. Mechanistic Understanding of Cellulose β-1,4-Glycosidic Cleavage via Photocatalysis. Appl. Catal. B Environ. 2022, 302, 120872. [Google Scholar] [CrossRef]
- Speltini, A.; Sturini, M.; Dondi, D.; Annovazzi, E.; Maraschi, F.; Caratto, V.; Profumo, A.; Buttafava, A. Sunlight-Promoted Photocatalytic Hydrogen Gas Evolution from Water-Suspended Cellulose: A Systematic Study. Photochem. Photobiol. Sci. 2014, 13, 1410–1419. [Google Scholar] [CrossRef]
- Han, G.; Jin, Y.H.; Burgess, R.A.; Dickenson, N.E.; Cao, X.M.; Sun, Y. Visible-Light-Driven Valorization of Biomass Intermediates Integrated with H2 Production Catalyzed by Ultrathin Ni/CdS Nanosheets. J. Am. Chem. Soc. 2017, 139, 15584–15587. [Google Scholar] [CrossRef]
- Low, J.; Yu, J.; Jaroniec, M.; Wageh, S.; Al-Ghamdi, A.A. Heterojunction Photocatalysts. Adv. Mater. 2017, 29, 1601694. [Google Scholar] [CrossRef]
- Wu, X.; Liu, C.; Li, X.; Zhang, X.; Wang, C.; Liu, Y. Effect of Morphology on the Photocatalytic Activity of G-C3N4 Photocatalysts under Visible-Light Irradiation. Mater. Sci. Semicond. Process. 2015, 32, 76–81. [Google Scholar] [CrossRef]
- Gong, J.; Imbault, A.; Farnood, R. The Promoting Role of Bismuth for the Enhanced Photocatalytic Oxidation of Lignin on Pt-TiO2 under Solar Light Illumination. Appl. Catal. B Environ. 2017, 204, 296–303. [Google Scholar] [CrossRef]
- Jongprateep, O.; Meesombad, K.; Techapiesancharoenkij, R.; Surawathanawises, K. Chemical Composition, Microstructure, Bandgap Energy and Electrocatalytic Activities of TiO2 and Ag-Doped TiO2 Powder Synthesized by Solution Combustion Technique. Ceram. Int. 2018, 44, S228–S232. [Google Scholar] [CrossRef]
- Marcì, G.; García-López, E.I.; Pomilla, F.R.; Palmisano, L.; Zaffora, A.; Santamaria, M.; Krivtsov, I.; Ilkaeva, M.; Barbieriková, Z.; Brezová, V. Photoelectrochemical and EPR Features of Polymeric C3N4 and O-Modified C3N4 Employed for Selective Photocatalytic Oxidation of Alcohols to Aldehydes. Catal. Today 2019, 328, 21–28. [Google Scholar] [CrossRef]
- Shiamala, L.; Alamelu, K.; Raja, V.; Jaffar Ali, B.M. Synthesis, Characterization and Application of TiO2–Bi2WO6 Nanocomposite Photocatalyst for Pretreatment of Starch Biomass and Generation of Biofuel Precursors. J. Environ. Chem. Eng. 2018, 6, 3306–3321. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Q.; Gao, Q.; Wan, S.; Yao, P.; Zhu, X. Preparation of Ag/β-Cyclodextrin Co-Doped TiO2 Floating Photocatalytic Membrane for Dynamic Adsorption and Photoactivity under Visible Light. Appl. Catal. B Environ. 2020, 267, 118715. [Google Scholar] [CrossRef]
- Wang, Y.; Lai, Q.; He, Y.; Fan, M. Selective Photocatalytic Carbon Dioxide Conversion with Pt@Ag-TiO2 Na-noparticles. Catal. Commun. 2018, 108, 98–102. [Google Scholar] [CrossRef]
- Preethi, L.K.; Mathews, T.; Nand, M.; Jha, S.N.; Gopinath, C.S.; Dash, S. Band Alignment and Charge Transfer Pathway in Three Phase Anatase-Rutile-Brookite TiO2 Nanotubes: An Efficient Photocatalyst for Water Splitting. Appl. Catal. B Environ. 2017, 218, 9–19. [Google Scholar] [CrossRef]
- Liu, X.; Dong, G.; Li, S.; Lu, G.; Bi, Y. Direct Observation of Charge Separation on Anatase TiO2 Crystals with Selectively Etched {001} Facets. J. Am. Chem. Soc. 2016, 138, 2917–2920. [Google Scholar] [CrossRef]
- Zhang, D.; Dong, S. Challenges in Band Alignment between Semiconducting Materials: A Case of Ru-tile and Anatase TiO2. Prog. Nat. Sci. Mater. Int. 2019, 29, 277–284. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Lin, W.; Li, Y.; Ding, K.N.; Li, J.Q. A Theoretical Study on the Electronic Structures of TiO2: Effect of Hartree−Fock Exchange. J. Phys. Chem. B 2005, 109, 19270–19277. [Google Scholar] [CrossRef]
- Momma, K.; Izumi, F. VESTA 3 for Three-Dimensional Visualization of Crystal, Volumetric and Morphology Data. J. Appl. Crystallogr. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
- Downs, R.; Hall-Wallace, M. American Mineralogist Crystal Structure Database. Am. Mineral. 2003, 88, 247–250. [Google Scholar]
- Kavan, L.; Grätzel, M.; Gilbert, S.E.; Klemenz, C.; Scheel, H.J. Electrochemical and Photoelectrochemical Investigation of Single-Crystal Anatase. J. Am. Chem. Soc. 1996, 118, 6716–6723. [Google Scholar] [CrossRef]
- Zhang, X.; Lin, Y.; He, D.; Zhang, J.; Fan, Z.; Xie, T. Interface Junction at Anatase/Rutile in Mixed-Phase TiO2: Formation and Photo-Generated Charge Carriers Properties. Chem. Phys. Lett. 2011, 504, 71–75. [Google Scholar] [CrossRef]
- Xiong, G.; Shao, R.; Droubay, T.C.; Joly, A.G.; Beck, K.M.; Chambers, S.A.; Hess, W.P. Photoemission Electron Microscopy of TiO2 Anatase Films Embedded with Rutile Nanocrystals. Adv. Funct. Mater. 2007, 17, 2133–2138. [Google Scholar] [CrossRef]
- Scanlon, D.O.; Dunnill, C.W.; Buckeridge, J.; Shevlin, S.A.; Logsdail, A.J.; Woodley, S.M.; Catlow, C.R.A.; Powell, M.J.; Palgrave, R.G.; Parkin, I.P.; et al. Band Alignment of Rutile and Anatase TiO2. Nat. Mater. 2013, 12, 798–801. [Google Scholar] [CrossRef]
- Ilkaeva, M.; Krivtsov, I.; García, J.R.; Díaz, E.; Ordóñez, S.; García-López, E.I.; Marcì, G.; Palmisano, L.; Mal-donado, M.I.; Malato, S. Selective Photocatalytic Oxidation of 5-Hydroxymethyl-2-Furfural in Aqueous Sus-pension of Polymeric Carbon Nitride and Its Adduct with H2O2 in a Solar Pilot Plant. Catal. Today 2018, 315, 138–148. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. A Critical Review on Graphitic Carbon Nitride (g-C3N4)-Based Materials: Preparation, Modification and Environmental Application. Coord. Chem. Rev. 2022, 453, 214338. [Google Scholar] [CrossRef]
- Kavitha, R.; Nithya, P.M.; Girish Kumar, S. Noble Metal Deposited Graphitic Carbon Nitride Based Hetero-junction Photocatalysts. Appl. Surf. Sci. 2020, 508, 145142. [Google Scholar] [CrossRef]
- Yan, D.; Wu, X.; Pei, J.; Wu, C.; Wang, X.; Zhao, H. Construction of G-C3N4/TiO2/Ag Composites with En-hanced Visible-Light Photocatalytic Activity and Antibacterial Properties. Ceram. Int. 2020, 46, 696–702. [Google Scholar] [CrossRef]
- Ma, J.; Yu, X.; Liu, X.; Li, H.; Hao, X.; Li, J. The Preparation and Photocatalytic Activity of Ag-Pd/g-C3N4 for the Coupling Reaction between Benzyl Alcohol and Aniline. Mol. Catal. 2019, 476, 110533. [Google Scholar] [CrossRef]
- Liang, H.; Wang, J.; Jin, B.; Li, D.; Men, Y. Direct Growth of Au Nanoparticles on g-C3N4 for Photo-catalytic Selective Alcohol Oxidations. Inorg. Chem. Commun. 2019, 109, 107574. [Google Scholar] [CrossRef]
- Nair, V.; Dhar, P.; Vinu, R. Production of Phenolics via Photocatalysis of Ball Milled Lignin–TiO2 Mixtures in Aqueous Suspension. RSC Adv. 2016, 6, 18204–18216. [Google Scholar] [CrossRef]
- Darabdhara, G.; Das, M.R. Bimetallic Au-Pd Nanoparticles on 2D Supported Graphitic Carbon Nitride and Reduced Graphene Oxide Sheets: A Comparative Photocatalytic Degradation Study of Organic Pollutants in Water. Chemosphere 2018, 197, 817–829. [Google Scholar] [CrossRef]
- Grabowska, E.; Marchelek, M.; Klimczuk, T.; Lisowski, W.; Zaleska-Medynska, A. Preparation, Characterization and Photocatalytic Activity of TiO2 Microspheres Decorated by Bimetallic Nanoparticles. J. Mol. Catal. A Chem. 2016, 424, 241–253. [Google Scholar] [CrossRef]
- Cybula, A.; Priebe, J.B.; Pohl, M.M.; Sobczak, J.W.; Schneider, M.; Zielińska-Jurek, A.; Brückner, A.; Zaleska, A. The Effect of Calcination Temperature on Structure and Photocatalytic Properties of Au/Pd Nanoparticles Supported on TiO2. Appl. Catal. B Environ. 2014, 152–153, 202–211. [Google Scholar] [CrossRef]
- Nguyen, T.T.H.; Le, M.C.; Ha, N.N. Understanding the Influence of Single Metal (Li, Mg, Al, Fe, Ag) Doping on the Electronic and Optical Properties of g-C3N4: A Theoretical Study. Mol. Simul. 2020, 47, 10–17. [Google Scholar] [CrossRef]
- Yang, C.; Zhang, G.; Meng, Y.; Pan, G.; Ni, Z.; Xia, S. Direct Z-Scheme CeO2@LDH Core–Shell Heterostructure for Photodegradation of Rhodamine B by Synergistic Persulfate Activation. J. Hazard. Mater. 2021, 408, 124908. [Google Scholar] [CrossRef]
- Raizada, P.; Kumar, A.; Hasija, V.; Singh, P.; Thakur, V.K.; Khan, A.A.P. An Overview of Converting Reductive Photocatalyst into All Solid-State and Direct Z-Scheme System for Water Splitting and CO2 Reduction. J. Ind. Eng. Chem. 2021, 93, 1–27. [Google Scholar] [CrossRef]
- Shao, Y.R.; Zhou, L.; Yu, L.; Li, Z.F.; Li, Y.T.; Li, W.; Hu, T.L. In Situ Construction of a Co/ZnO@C Heterojunction Catalyst for Efficient Hydrogenation of Biomass Derivative under Mild Conditions. ACS Appl. Mater. Interfaces 2022, 14, 17195–17207. [Google Scholar] [CrossRef]
- Chen, S.; Qian, T.T.; Ling, L.L.; Zhang, W.; Gong, B.B.; Jiang, H. Hydrogenation of Furfural to Cyclopentanone under Mild Conditions by a Structure-Optimized Ni−NiO/TiO2 Heterojunction Catalyst. ChemSusChem 2020, 13, 5507–5515. [Google Scholar] [CrossRef]
- Dutta, S.; Bhat, N.S. Catalytic Transformation of Biomass-Derived Furfurals to Cyclopentanones and Their Derivatives: A Review. ACS Omega 2021, 6, 35145–35172. [Google Scholar] [CrossRef]
- Fernández, M.A.; Silva, O.F.; Vico, R.V.; de Rossi, R.H. Complex Systems That Incorporate Cyclodextrins to Get Materials for Some Specific Applications. Carbohydr. Res. 2019, 480, 12–34. [Google Scholar] [CrossRef]
- Espinoza-Villalobos, N.; Rojas, S.; Salazar, R.A.; Contreras, D.; Escalona, N.; Vergara, E.; Laguna-Bercero, M.A.; Mendizabal, F.; Barrientos, L. Role of β-CD Macromolecule Anchored to α-Fe2O3/TiO2 on the Selectivity and Partial Oxidation of Guaiacol to Add-Value Products. ACS Sustain. Chem. Eng. 2021, 9, 11427–11438. [Google Scholar] [CrossRef]
- Lannoy, A.; Kania, N.; Bleta, R.; Fourmentin, S.; Machut-Binkowski, C.; Monflier, E.; Ponchel, A. Photocatalysis of Volatile Organic Compounds in Water: Towards a Deeper Understanding of the Role of Cyclodextrins in the Photodegradation of Toluene over Titanium Dioxide. J. Colloid Interface Sci. 2016, 461, 317–325. [Google Scholar] [CrossRef]
- Liu, Q.; Zhou, Y.; Lu, J.; Zhou, Y. Novel Cyclodextrin-Based Adsorbents for Removing Pollutants from Wastewater: A Critical Review. Chemosphere 2020, 241, 125043. [Google Scholar] [CrossRef]
- Crumling, M.A.; King, K.A.; Duncan, R.K. Cyclodextrins and Iatrogenic Hearing Loss: New Drugs with Significant Risk. Front. Cell. Neurosci. 2017, 11, 355. [Google Scholar] [CrossRef]
- Chen, X.; Liu, D.; Wu, Z.; Cravotto, G.; Wu, Z.; Ye, B.C. Microwave-Assisted Rapid Synthesis of Ag-β-Cyclodextrin/TiO2/A.C. with Exposed {001} Facets for Highly Efficient Naphthalene Degradation under Visible Light. Catal. Commun. 2018, 104, 96–100. [Google Scholar] [CrossRef]
- Yasuda, M.; Miura, A.; Yuki, R.; Nakamura, Y.; Shiragami, T.; Ishii, Y.; Yokoi, H. The Effect of TiO2-Photocatalytic Pretreatment on the Biological Production of Ethanol from Lignocelluloses. J. Photochem. Photobiol. A Chem. 2011, 220, 195–199. [Google Scholar] [CrossRef]
- Zhang, G.; Ni, C.; Huang, X.; Welgamage, A.; Lawton, L.A.; Robertson, P.K.J.; Irvine, J.T.S. Simultaneous Cellulose Conversion and Hydrogen Production Assisted by Cellulose Decomposition under UV-Light Photoca-talysis. Chem. Commun. 2016, 52, 1673–1676. [Google Scholar] [CrossRef]
- Yin, J.; Zhang, Q.; Yang, C.; Zhang, B.; Deng, K. Highly Selective Oxidation of Glucose to Gluconic Acid and Glucaric Acid in Water Catalyzed by an Efficient Synergistic Photocatalytic System. Catal. Sci. Technol. 2020, 10, 2231–2241. [Google Scholar] [CrossRef]
- Jin, B.; Yao, G.; Wang, X.; Ding, K.; Jin, F. Photocatalytic Oxidation of Glucose into Formate on Nano TiO2 Catalyst. ACS Sustain. Chem. Eng. 2017, 5, 6377–6381. [Google Scholar] [CrossRef]
- Chong, R.; Li, J.; Ma, Y.; Zhang, B.; Han, H.; Li, C. Selective Conversion of Aqueous Glucose to Value-Added Sugar Aldose on TiO2-Based Photocatalysts. J. Catal. 2014, 314, 101–108. [Google Scholar] [CrossRef]
- Navakoteswara Rao, V.; Malu, T.J.; Cheralathan, K.K.; Sakar, M.; Pitchaimuthu, S.; Rodríguez-González, V.; Mamatha Kumari, M.; Shankar, M.V. Light-Driven Transformation of Biomass into Chemicals Using Photo-catalysts—Vistas and Challenges. J. Environ. Manag. 2021, 284, 111983–111994. [Google Scholar] [CrossRef]
- Bellardita, M.; García-López, E.I.; Marcì, G.; Palmisano, L. Photocatalytic Formation of H2 and Value-Added Chemicals in Aqueous Glucose (Pt)-TiO2 Suspension. Int. J. Hydrogen Energy 2016, 41, 5934–5947. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, Z.; Zhang, L.; Xue, S.; Doherty, W.O.S.; O’Hara, I.M.; Ke, X. Sustainable Conversion of Cellulosic Biomass to Chemicals under Visible-Light Irradiation. RSC Adv. 2015, 5, 85242–85247. [Google Scholar] [CrossRef]
- Zou, J.; Zhang, G.; Xu, X. One-Pot Photoreforming of Cellulosic Biomass Waste to Hydrogen by Merging Photocatalysis with Acid Hydrolysis. Appl. Catal. A Gen. 2018, 563, 73–79. [Google Scholar] [CrossRef]
- Kim, S.; Chmely, S.C.; Nimlos, M.R.; Bomble, Y.J.; Foust, T.D.; Paton, R.S.; Beckham, G.T. Computational Study of Bond Dissociation Enthalpies for a Large Range of Native and Modified Lignins. J. Phys. Chem. Lett. 2011, 2, 2846–2852. [Google Scholar] [CrossRef]
- Gazi, S.; Hung Ng, W.K.; Ganguly, R.; Putra Moeljadi, A.M.; Hirao, H.; Soo, H. Sen Selective Photocatalytic C-C Bond Cleavage under Ambient Conditions with Earth Abundant Vanadium Complexes. Chem. Sci. 2015, 6, 7130–7142. [Google Scholar] [CrossRef]
- Kamwilaisak, K.; Wright, P.C. Investigating Laccase and Titanium Dioxide for Lignin Degradation. Energy Fuels 2012, 26, 2400–2406. [Google Scholar] [CrossRef]
- KäRkä, M.D.; Bosque, I.; Matsuura, B.S.; Stephenson, C.R.J. Photocatalytic Oxidation of Lignin Model Systems by Merging Visible-Light Photoredox and Palladium Catalysis. Org. Lett. 2016, 18, 5166–5169. [Google Scholar] [CrossRef]
- Ahmad, K.; Roy Ghatak, H.; Ahuja, S.M. Kinetics of Producing Vanillin and 4-Hydroxy Benzaldehyde from the Hydrolysis Residue of Rice Straw by Photocatalysis. React. Kinet. Mech. Catal. 2020, 131, 383–395. [Google Scholar] [CrossRef]
- Srisasiwimon, N.; Chuangchote, S.; Laosiripojana, N.; Sagawa, T. TiO2/Lignin-Based Carbon Composited Pho-tocatalysts for Enhanced Photocatalytic Conversion of Lignin to High-Value Chemicals. ACS Sustain. Chem. Eng. 2018, 6, 13968–13976. [Google Scholar] [CrossRef]
- Tian, M.; Wen, J.; MacDonald, D.; Asmussen, R.M.; Chen, A. A Novel Approach for Lignin Modification and Degradation. Electrochem. Commun. 2010, 12, 527–530. [Google Scholar] [CrossRef]

















| Catalyst | Metal | Bandgap (eV) |
|---|---|---|
| g-C3N4 | - | 2.88 |
| Ag | 2.86 | |
| Pt | 2.7 | |
| Pd | 2.78 | |
| Au | 2.79 | |
| TiO2 | - | 3.23 |
| Ag | 3.17 | |
| Pt | 3.08 | |
| Pd | 3.05 | |
| Au | 3.1 |
| Photocatalyst | Reactive | Product | % Conversion | % Selectivity | Ref. |
|---|---|---|---|---|---|
| g-C3N4 | Benzyl | Imine | 5.7 | >99 | [111] |
| Ag/g-C3N4 (1%) | alcohol | 12.3 | |||
| Pd/g-C3N4 (1%) | 31.7 | ||||
| Ag-Pd/g-C3N4 (1%) | 31.4 | ||||
| Ag-Pd/g-C3N4 (2%) | 58.9 | ||||
| TiO2 | CO2 | CH4 | 50 | 42.11 | [96] |
| Pt/TiO2 | 74.21 | ||||
| Ag/TiO2 | 84.52 | ||||
| Pt-Ag/TiO2 | 87.9 |
| Substrate | Photocatalyst | Light | Conversion (%) | Products | Ref. |
|---|---|---|---|---|---|
| Glucose | TiO2 | Mercury lamp (400 W) | 6.45 | Xylitol Gluconic acid | [9] |
| Cellulose | CdS/CdOx | Simulated solar light | 9.7 | H2 | [85] |
| Cellulose | Au-HYT | Visible light | 59 | Glucose HMF | [136] |
| Cellulose | Pt-TiO2(P25) | U.V. | 66 | Arabinose Erythrose HMF H2 | [137] |
| Substrate | Photocatalyst | Light Source | Conversión (%) | Main Products | Ref. |
|---|---|---|---|---|---|
| Lignosulfonate | Bi1%/Pt15-TiO2 | Xe lamp (300 W) | 84.5 | Guaiacol | [91] |
| Lignin | TiO2 Lacasse H2O2 | UV irradiation (24 W) | 100 | Organic acids Fatty acids Carbohydrates | [140] |
| Kraft lignin | TiO2/carbon | Mercury lamp (400 W) | 40.3 | Vanillin | [143] |
| Kraft lignin | TiO2/Ti/Ta2O5-IrO2 | Blue wave TM 50 AS UV spot lamp | 92 | Vanillin Vanillic acid | [144] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Llatance-Guevara, L.; Flores, N.E.; Barrionuevo, G.O.; Mullo Casillas, J.L. Waste Biomass Selective and Sustainable Photooxidation to High-Added-Value Products: A Review. Catalysts 2022, 12, 1091. https://doi.org/10.3390/catal12101091
Llatance-Guevara L, Flores NE, Barrionuevo GO, Mullo Casillas JL. Waste Biomass Selective and Sustainable Photooxidation to High-Added-Value Products: A Review. Catalysts. 2022; 12(10):1091. https://doi.org/10.3390/catal12101091
Chicago/Turabian StyleLlatance-Guevara, Liliana, Nelly Esther Flores, Germán Omar Barrionuevo, and José Luis Mullo Casillas. 2022. "Waste Biomass Selective and Sustainable Photooxidation to High-Added-Value Products: A Review" Catalysts 12, no. 10: 1091. https://doi.org/10.3390/catal12101091