Highly Efficient and Ambient-Temperature Synthesis of Benzimidazoles via Co(III)/Co(II)-Mediated Redox Catalysis
Abstract
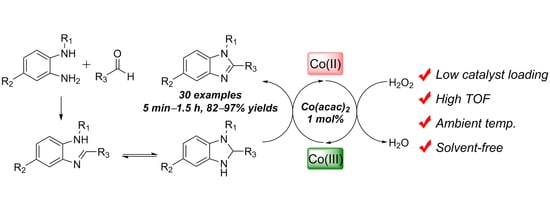
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. General Methods
3.2. General Synthetic Procedure and Characterization of Benzimidazoles
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bansal, Y.; Silakari, O. The therapeutic journey of benzimidazoles: A review. Bioorg. Med. Chem. 2012, 20, 6208–6236. [Google Scholar] [CrossRef]
- Aili, D.; Yang, J.; Jankova, K.; Henkensmeier, D.; Li, Q. From polybenzimidazoles to polybenzimidazoliums and polybenzimidazolides. J. Mater. Chem. A 2020, 8, 12854–12886. [Google Scholar] [CrossRef]
- Keri, R.S.; Hiremathad, A.; Budagumpi, S.; Nagaraja, B.M. Comprehensive review in current developments of benzimidazole-based medicinal chemistry. Chem. Biol. Drug Des. 2015, 86, 19–65. [Google Scholar] [CrossRef]
- Sreerama, R.; Barnali, M.; Balamurali, M.M.; Chanda, K. Synthesis and medicinal applications of benzimidazoles: An overview. Curr. Org. Synth. 2017, 14, 40–60. [Google Scholar]
- Yadav, G.; Ganguly, S. Structure activity relationship (SAR) study of benzimidazole scaffold for different biological activities: A mini-review. Eur. J. Med. Chem. 2015, 46, 419–443. [Google Scholar] [CrossRef]
- Vasava, M.S.; Bhoi, M.N.; Rathwa, S.K.; Jethava, D.J.; Acharya, P.T.; Patel, D.B.; Patel, H.D. Benzimidazole: A milestone in the field of medicinal chemistry. Mini Rev. Med. Chem. 2020, 20, 532–565. [Google Scholar] [CrossRef]
- Bai, Y.-B.; Zhang, A.-L.; Tang, J.-J.; Gao, J.-M. Synthesis and antifungal activity of 2-chloromethyl-1H-benzimidazole derivatives against phytopathogenic fungi in vitro. J. Agric. Food Chem. 2013, 61, 2789–2795. [Google Scholar] [CrossRef]
- Ballari, M.S.; Cano, N.H.; Lopez, A.G.; Wunderlin, D.A.; Feresín, G.E.; Santiago, A.N. Green synthesis of potential antifungal agents: 2-Benzyl substituted thiobenzoazoles. J. Agric. Food Chem. 2017, 65, 10325–10331. [Google Scholar] [CrossRef]
- Carvalho, L.C.R.; Fernandes, E.; Marques, M.M.B. Developments towards regioselective synthesis of 1,2-disubstituted benzimidazoles. Chem. Eur. J. 2011, 17, 12544–12555. [Google Scholar] [CrossRef]
- Faheem, M.; Rathaur, A.; Pandey, A.; Singh, K.V.; Tiwari, A.K. A review on the modern synthetic approach of benzimidazole candidate. ChemistrySelect 2020, 5, 3981–3994. [Google Scholar] [CrossRef]
- Mamedov, V.A.; Zhukova, N.A. Recent developments towards synthesis of (het)arylbenzimidazoles. Synthesis 2021, 53, 1849–1878. [Google Scholar] [CrossRef]
- Itoh, T.; Nagata, K.; Ishikawa, H.; Ohsawa, A. Synthesis of 2-arylbenzothiazoles and imidazoles using scandium triflate as a catalyst for both a ring closing and an oxidation steps. Heterocycles 2004, 63, 2769–2783. [Google Scholar] [CrossRef]
- Nagawade, R.R.; Shinde, D.B. Zirconyl(IV) chloride-promoted synthesis of benzimidazole derivatives. Russ. J. Org. Chem. 2006, 42, 453–454. [Google Scholar] [CrossRef]
- Shen, M.-G.; Cai, C. Ytterbium perfluorooctanesulfonates catalyzed synthesis of benzimidazole derivatives in fluorous solvents. J. Fluor. Chem. 2007, 128, 232–235. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; Kanagaraj, K.; Pitchumani, K. Zn2+-K10-clay (clayzic) as an efficient water-tolerant, solid acid catalyst for the synthesis of benzimidazoles and quinoxalines at room temperature. Tetrahedron Lett. 2011, 52, 69–73. [Google Scholar] [CrossRef]
- Jayabharathi, J.; Thanikachalam, V.; Jayamoorthy, K. Synthesis of some fluorescent benzimidazole derivatives using cobalt(II) hydroxide as highly efficient catalyst–spectral and physico-chemical studies and ESIPT process. Photochem. Photobiol. Sci. 2013, 12, 1761–1773. [Google Scholar] [CrossRef]
- Martins, G.M.; Puccinelli, T.; Gariani, R.A.; Xavier, F.R.; Silveira, C.C.; Mendes, S.R. Facile and efficient aerobic one-pot synthesis of benzimidazoles using Ce(NO3)3·6H2O as promoter. Tetrahedron Lett. 2017, 58, 1969–1972. [Google Scholar] [CrossRef]
- Kommi, D.N.; Jadhavar, P.S.; Kumar, D.; Chakraborti, A.K. “All-water” one-pot diverse synthesis of 1,2-disubstituted benzimidazoles: Hydrogen bond driven synergistic electrophile–nucleophile dual activation by water. Green Chem. 2013, 15, 798–810. [Google Scholar] [CrossRef]
- Peng, X.-C.; Gong, S.-S.; Zeng, D.-Y.; Duo, S.-W.; Sun, Q. Activated carbon supported hafnium(IV) chloride as an efficient, recyclable, and facile removable catalyst for expeditious parallel synthesis of benzimidazoles. Catalysts 2020, 10, 436. [Google Scholar] [CrossRef] [Green Version]
- Bonacci, S.; Iriti, G.; Mancuso, S.; Novelli, P.; Paonessa, R.; Tallarico, S.; Nardi, M. Montmorillonite K10: An efficient organo-heterogeneous catalyst for synthesis of benzimidazole derivatives. Catalysts 2020, 10, 845. [Google Scholar] [CrossRef]
- Gioia, M.L.D.; Cassano, R.; Costanzo, P.; Cano, N.H.; Maiuolo, L.; Nardi, M.; Nicoletta, F.P.; Oliverio, M.; Procopio, A. Green synthesis of privileged benzimidazole scaffolds using active deep eutectic solvent. Molecules 2019, 24, 2885. [Google Scholar] [CrossRef] [Green Version]
- Beaulieu, P.L.; Haché, B.; Moos, E.V. A practical Oxone®–mediated, high-throughput, solution-phase synthesis of benzimidazoles from 1,2-phenylenediamines and aldehydes and its application to preparative scale synthesis. Synthesis 2003, 11, 1683–1692. [Google Scholar] [CrossRef] [Green Version]
- Verner, E.; Katz, B.A.; Spencer, J.R.; Allen, D.; Hataye, J.; Hruzewicz, W.; Hui, H.C.; Kolesnikov, A.; Li, Y.; Luong, C.; et al. Development of serine protease inhibitors displaying a multicentered short (<2.3 Å) hydrogen bond binding mode: Inhibitors of urokinase-type plasminogen activator and factor Xa. J. Med. Chem. 2001, 44, 2753–2771. [Google Scholar] [CrossRef]
- Du, L.-H.; Wang, Y.-G. A rapid and efficient synthesis of benzimidazoles using hypervalent iodine as oxidant. Synthesis 2007, 5, 675–678. [Google Scholar] [CrossRef]
- Hu, Z.; Zhao, T.; Wang, M.; Wu, J.; Yu, W.; Chang, J. I2-mediated Intramolecular C–H amidation for the synthesis of N-substituted benzimidazoles. J. Org. Chem. 2017, 82, 3152–3158. [Google Scholar] [CrossRef]
- Sun, Q.; Wang, C.-J.; Gong, S.-S.; Ai, Y.-J.; Sun, H.-B. Cp2ZrCl2-catalyzed synthesis of 2-aminovinyl benzimidazoles under microwave conditions. Chin. Chem. Lett. 2015, 26, 297–300. [Google Scholar] [CrossRef]
- Alapati, M.L.P.R.; Abburi, S.R.; Mukkamala, S.B.; Rao, M.K. A simple and efficient one-pot synthesis of 2-substituted benzimidazoles from Ɵ-diaminoarene and aryl aldehydes. Synth. Commun. 2015, 45, 2436–2443. [Google Scholar] [CrossRef]
- Bahrami, K.; Khodaei, M.M.; Naali, F. Mild and highly efficient method for the synthesis of 2-arylbenzimidazoles and 2-arylbenzothiazoles. J. Org. Chem. 2008, 73, 6835–6837. [Google Scholar] [CrossRef]
- Nasr-Esfahani, M.; Mohammadpoor-Baltork, I.; Khosropour, A.R.; Moghadam, M.; Mirkhani, V.; Tangestaninejad, S. Synthesis and characterization of Cu(II)-containing nanosilica triazine dendrimer: A recyclable nanocomposite material for the synthesis of benzimidazoles, benzothiazoles, bisbenzimidazoles and bisbenzothiazoles. J. Mol. Catal. A Chem. 2013, 379, 243–254. [Google Scholar] [CrossRef]
- Roy, S.; Banerjee, B.; Salam, N.; Bhaumik, A.; Islam, S.M. Mesoporous titania-iron(III) oxide with nanoscale porosity and high catalytic activity for the synthesis of β-Aminoalcohols and benzimidazole derivatives. ChemCatChem 2015, 7, 2689–2697. [Google Scholar] [CrossRef]
- Dandia, A.; Parewa, V.; Rathore, K.S. Synthesis and characterization of CdS and Mn doped CdS nanoparticles and their catalytic application for chemoselective synthesis of benzimidazoles and benzothiazoles in aqueous medium. Catal. Commun. 2012, 28, 90–94. [Google Scholar] [CrossRef]
- Bai, G.; Lan, X.; Liu, X.; Liu, C.; Shi, L.; Chen, Q.; Chen, G. An ammonium molybdate deposited amorphous silica coated iron oxide magnetic core–shell nanocomposite for the efficient synthesis of 2-benzimidazoles using hydrogen peroxide. Green Chem. 2014, 16, 3160–3168. [Google Scholar] [CrossRef]
- Karimian, A.; Kakhki, R.M.; Beidokhti, H.K. Magnetic Co-doped NiFe2O4 nanocomposite: A heterogeneous and recyclable catalyst for the one-pot synthesis of benzimidazoles, benzoxazoles andbenzothiazoles under solvent-free conditions. J. Chin. Chem. Soc. 2017, 64, 1316–1325. [Google Scholar] [CrossRef]
- Xiong, W.-L.; Peng, X.-C.; Zhong, R.-Y.; Zheng, J.; Duo, S.; Gong, S.-S.; Sun, H.-B.; Sun, Q. Construction of a clock catalytic system: Highly efficient and self-Indicating synthesis of benzoheterocycles at ambient temperature. Asian J. Org. Chem. 2021, 10, 3321–3327. [Google Scholar] [CrossRef]
- Cano, N.H.; Uranga, J.G.; Nardi, M.; Procopio, A.; Wunderlin, D.A.; Santiago, A.N. Selective and eco-friendly procedures for the synthesis of benzimidazole derivatives. The role of the Er(OTf)3 catalyst in the reaction selectivity. Beilstein J. Org. Chem. 2016, 12, 2410–2419. [Google Scholar] [CrossRef] [PubMed] [Green Version]

 | |||
|---|---|---|---|
| Entry | Solvent | Reaction Time (h) | Isolated Yield (%) |
| 1 | DMSO | 2.5 | 72 2 |
| 2 | THF | 1.5 | 79 2 |
| 3 | DME | 1.25 | 82 2 |
| 4 | CH3CN | 40 min | 80 2 |
| 5 | EtOH | 20 min | 81 2 |
| 6 | Solvent-free | 15 min | 90 |
 | |||
|---|---|---|---|
| Entry | Catalyst | Reaction Time (min) | Isolated Yield (%) |
| 1 | Cu(acac)2 | 2 | 78 |
| 2 | Ni(acac)2 | 30 | 87 |
| 3 | MoO2(acac)2 | 15 | 90 |
| 4 | VO(acac)2 | 15 | 89 |
| 5 | Fe(acac)3 | 10 | 91 |
| 6 | Ce(acac)3 | 10 | 92 |
| 7 | Co(acac)3 | 7 | 97 |
| 8 | Co(acac)2 | 7 | 97 |
| 9 | Co(OAc)2 | 10 | 92 |
| 10 | CoCl2 | 15 | 90 |
| 11 | No catalyst | 360 | 82 |
 | ||||
|---|---|---|---|---|
| Entry | Oxidant | Co(acac)2 (mol%) | Reaction Time (min) | Isolated Yield (%) |
| 1 | mCPBA | 1 | 5 | 54 |
| 2 | UHP | 1 | 30 | 85 |
| 3 | TBHP (70% aq.) | 1 | 15 | 94 |
| 4 | TBHP (5.5 M in decane) | 1 | 7 | 97 |
| 5 | H2O2 | 1 | 5 | 97 |
| 6 | H2O2 | 2 | 5 | 90 |
| 7 | H2O2 | 0.5 | 15 | 95 |
| 8 | No peroxide (air only) | 1 | 24 h | 88 |
 |
|---|
 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhong, R.; Xiong, W.; Zhang, H.; Zeng, T.; Gong, S.; Sun, Q. Highly Efficient and Ambient-Temperature Synthesis of Benzimidazoles via Co(III)/Co(II)-Mediated Redox Catalysis. Catalysts 2022, 12, 59. https://doi.org/10.3390/catal12010059
Zhong R, Xiong W, Zhang H, Zeng T, Gong S, Sun Q. Highly Efficient and Ambient-Temperature Synthesis of Benzimidazoles via Co(III)/Co(II)-Mediated Redox Catalysis. Catalysts. 2022; 12(1):59. https://doi.org/10.3390/catal12010059
Chicago/Turabian StyleZhong, Renyuan, Wulin Xiong, Haoyuan Zhang, Tongtong Zeng, Shanshan Gong, and Qi Sun. 2022. "Highly Efficient and Ambient-Temperature Synthesis of Benzimidazoles via Co(III)/Co(II)-Mediated Redox Catalysis" Catalysts 12, no. 1: 59. https://doi.org/10.3390/catal12010059