Development of Pilot-Scale CO2 Methanation Using Pellet-Type Catalysts for CO2 Recycling in Sewage Treatment Plants and Its Validation through Computational Fluid Dynamics (CFD) Modeling
Abstract
:1. Introduction
2. Results and Discussion
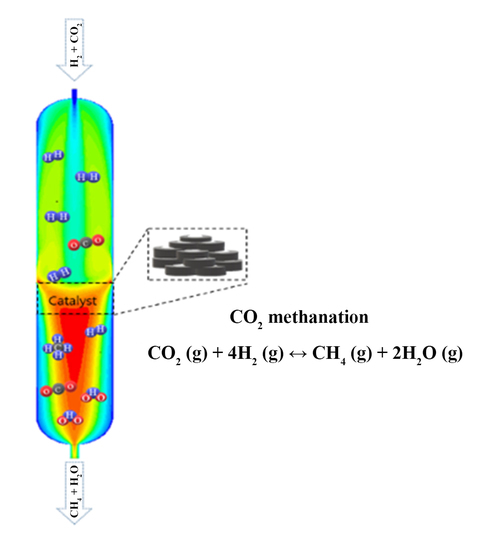
2.1. CFD Results for the CO2 Methanation Reactor
2.1.1. Cold-Flow Simulation
2.1.2. Reacting-Flow Simulation
2.1.3. Flow Uniformity
2.2. Pilot Plant Operating Real Data (CO2 Methanation)
2.3. CO2 Methanation-System Energy Balance
3. Experimental and Method
3.1. Pilot Plant Description
3.1.1. CO2 Methanation Technology Applied to Sewage Disposal Plants
3.1.2. Pilot Scale Fixed Bed Methanation System
3.2. Materials and Methods
3.2.1. Preparation of Pellet Catalysts
3.2.2. Operating Setup of the Pilot Scale Plant
3.3. CFD Approach for Reactor Design of CO2 Methanation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Norbert, E.; Duchel, S.; Sung Jin, K.; Andreas, O.; Sun Jin, Y.; Gi Eun, K. KAS Journal on Contemporary Korean Affairs, Environmental Policy in South Korea: Problems and Perspectives; Konrad Adenauer Stiftung: Seoul, Korea, 2015; pp. 45–48. [Google Scholar]
- European Commission. A Policy Framework for the Climate and Energy in the Period from 2020 to 2030. 2014. Available online: https://ec.europa.eu/clima/policies/strategies/2030_en (accessed on 20 August 2021).
- Frédéric David, M.; Frédéric-Paul, P.; Suren, E. Power-to-gas through CO2 methanation: Assessment of the carbon balance regarding EU directives. J. Energy Storage 2017, 11, 16–24. [Google Scholar]
- Rocha, A.; Pereira, S.C.; Viceto, C.; Silva, R.; Neto, J.; Marta Almeida, M. A consistent methodology to evaluate temperature and heat wave future projections for cities: A case study for Lisbon. Appl. Sci. 2020, 10, 1149. [Google Scholar] [CrossRef] [Green Version]
- Wei, Z.; Hiroshi, M.; Hiroyuki, T.; Koichi, I.; Koyo, N. Computational fluid dynamics simulation of CO2 methanation in a shell-and-tube reactor with multi-region conjugate heat transfer. Chem. Eng. Sci. 2020, 211, 115–276. [Google Scholar]
- Yamasaki, M.; Habazaki, H.; Yoshida, T.; Akiyama, E.; Kawashima, A.; Asami, K.; Komori, M.; Shimamura, K. Compositional dependence of the CO2 methanation activity of Ni/ZrO2 catalysts prepared from amorphous Ni-Zr alloy precursors. Appl. Catal. A Gen. 1997, 163, 187–197. [Google Scholar] [CrossRef]
- Götz, M.; Lefebvre, J.; Mörs, F.; McDaniel, K.; Graf, F.; Bajohr, S. Renewable Power-to-Gas: A Technological and Economic Review. Renew. Energy 2016, 85, 1371–1390. [Google Scholar] [CrossRef] [Green Version]
- Coronado Martín, I. Carbon Dioxide Methanation for Intensified Reactors. In Proceedings of the Aalto University School of Chemical Technology Degree Programme in Chemical Technology, Helsinki, Finland, 9 March 2015. [Google Scholar]
- Beatrice, C.; Alberto, M.G.; Elena, M.; Benedetto, N.; Andrea, P.; Mirko, F.; Andrea, N.; Federico, R. Experimental Investigation on CO2 Methanation Process for Solar Energy Storage Compared to CO2-Based Methanol Synthesis. Energies 2017, 10, 855. [Google Scholar]
- Shohei, T.; Teruyuki, S.; Hiromichi, K.; Takahide, H.; Ryuji, K. Ni/CeO2 catalysts with high CO2 methanation activity and high CH4 selectivity at low temperatures. Int. J. Hydrog. Energy 2012, 37, 5527–5531. [Google Scholar]
- Han, D.; Kim, Y.; Byun, H.; Cho, W.; Baek, Y. CO2 methanation of biogas over 20 wt% Ni-Mg-Al catalyst: On the effect of N2, CH4, and O2 on CO2 conversion rate. Catalysts 2020, 10, 1201. [Google Scholar] [CrossRef]
- Pastor Pérez, L.; Baibars, F.; Le Sache, E.; Arellano García, H.; Gu, S.; Reina, T.R. CO2 valorisation via Reverse Water-Gas Shift reaction using advanced Cs doped Fe-Cu/Al2O3 catalysts. J. CO2 Util. 2017, 21, 423–428. [Google Scholar] [CrossRef] [Green Version]
- Jinghua, X.; Xiong, S.; Hongmin, D.; Baolin, H.; Qingquan, L.; Xiaoyan, L.; Xiaoli, P.; Guangxian, P.; Haoran, G.; Yanqiang, H.; et al. Influence of pretreatment temperature on catalytic performance of rutile TiO2-supported ruthenium catalyst in CO2 methanation. J. Catal. 2016, 333, 227–237. [Google Scholar]
- Beuls, A.; Swalus, C.; Jacquemin, M.; Heyen, G.; Karelovic, A.; Ruiz, P. Methanation of CO2: Further insight into the mechanism over Rh/γ-Al2O3 catalyst. Appl. Catal. B Environ. 2012, 113–114, 2–10. [Google Scholar] [CrossRef]
- Takano, H.; Shinomiya, H.; Izumiya, K.; Kumagai, N.; Habazaki, H.; Hashimoto, K. CO2 methanation of Ni catalysts supported on tetragonal ZrO2 doped with Ca2+ and Ni2+ ions. Int. J. Hydrog. Energy 2015, 40, 8347–8355. [Google Scholar] [CrossRef]
- Rynkowski, J.; Paryjczak, T.; Lewicki, A.; Szynkowska, M.I.; Maniecki, T.P.; Jozwiak, W.K. Characterization of Ru/CeO2-Al2O3 catalysts and their performance in CO2 methanation. React. Kinet. Catal. Lett. 2000, 71, 55–64. [Google Scholar] [CrossRef]
- Dea Heun, M.; Woo Jin, C.; Soon Woong, C.; Sang Moon, L.; Sung Su, K.; Jae Hoon, J.; Yeon Hee, R.; Jeong Yoon, A.; Wenshan, G.; Huu Hao, N.; et al. Fabrication and characterization of Ni-Ce-Zr ternary disk-shaped catalyst and its application for low-temperature CO2 methanation. Fuel 2020, 260, 116–260. [Google Scholar]
- Jeong Yoon, A.; Soon Woong, C.; Sang Moon, L.; Sung Su, K.; Woo Jin, C.; Jung Chul, L.; Yong Joo, C.; Kyung Sook, S.; Dea Heun, M.; Dinh Duc, N. Developing Ni-based honeycomb-type catalysts using different binary oxide-supported species for synergistically enhanced CO2 methanation activity. Fuel 2019, 250, 227–284. [Google Scholar]
- Dea Heun, M.; Sang Moon, L.; Jeong Yoon, A.; Dinh Duc, N.; Sung Su, K.; Soon Woong, C. New Ni-based Quaternary Disk-Shaped Catalysts for Low-Temperature CO2 Methanation: Fabrication, Characterization, and Performance. J. Environ. Manag. 2018, 218, 88–94. [Google Scholar]
- Yu Jin, J.; Sung Gil, H.; Min Choul, K.; Jae Jeong, L.; Gang Woo, L.; Byung Hyun, S. Numerical Study for Flow Uniformity in Selective Catalytic Reduction(SCR) Process. J. Korea Acad. Ind. Coop. Soc. 2011, 12, 4666–4672. [Google Scholar]
- Hey Suk, K.; Seung Hee, C.; Min Jung, H.; Woo Young, S.; Mi Soo, S.; Dong Soon, J.; Sang June, Y.; Young Chan, C.; Gae Goo, L. Numerical and Experimental Study on the Coal Reaction in an Entrained Flow Gasifier. J. Korean Soc. Environ. Eng. 2010, 32, 165–174. [Google Scholar]
- Mi Soo, S.; Hey Suk, K.; Ji Eun, H.; Dong Soon, J. A numerical Study for improvement of indoor Air Quality of Apartment House. J. Korean Soc. Environ. Eng. 2009, 31, 521–530. [Google Scholar]
- Lars, K.; Jorg, T. Predicting optimal temperature profiles in single-stage fixed-bed reactors for CO2-methanation. Chem. Eng. Sci. 2016, 132, 59–71. [Google Scholar]
- Gonzatti, F.; Nizolli, V.; Ferrigolo, F.Z.; Farret, F.A.; de Mello, M.A.S. Experimental Hydrogen Plant with Metal Hydrides to Store and Generate Electrical Power. Int. J. Emerg. Electr. Power Syst. 2016, 17, 59–67. [Google Scholar] [CrossRef]
- Frédéric, D.M.; Vincent, M.; Suren, E. Material constraints related to storage of future European renewable electricity surpluses with CO2 methanation. Energy Policy 2016, 94, 366–376. [Google Scholar]














| Reactor Type | A | B | |
|---|---|---|---|
| Exit-gas temperature (°C) | 497 | 484 | |
| Exit-gas compound (vol%) | CO2 | 6.78 | 3.75 |
| CH4 | 22.05 | 27.15 | |
| Unit | Data | |
|---|---|---|
| CO2 | Nm3/day | 50 |
| Capture CO2 | kWh/ton CO2 | 1320 |
| CO2-methanation system | kWh/ton CO2 | 1100 |
| CH4 gas | Nm3/day | 43.3 |
| Chemical energy (CH4) | kWh/ton CO2 | 4389 |
| Recoverable heat | kWh/ton CO2 | 1038 |
| Experimental Conditions | ||
|---|---|---|
| Flow rate | H2 (Nm3/h) | 8.33 |
| CO2 (Nm3/h) | 2.08 | |
| Average working temperature (°C) | 300 | |
| Max working temperature | 360 | |
| Catalyst type | pellet type Ni–0.2Ce–0.1Zr | |
| Space velocity (h−1) | 1950 | |
| Working pressure (bar) | 1–1.2 | |
| Number of grid (Cells) | Type 1 | 127,348 |
| Type 2 | 157,095 | |
| mesh type | Polyhedral mesh | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahn, J.; Kim, H.; Ro, Y.; Kim, J.; Chung, W.; Chang, S. Development of Pilot-Scale CO2 Methanation Using Pellet-Type Catalysts for CO2 Recycling in Sewage Treatment Plants and Its Validation through Computational Fluid Dynamics (CFD) Modeling. Catalysts 2021, 11, 1005. https://doi.org/10.3390/catal11081005
Ahn J, Kim H, Ro Y, Kim J, Chung W, Chang S. Development of Pilot-Scale CO2 Methanation Using Pellet-Type Catalysts for CO2 Recycling in Sewage Treatment Plants and Its Validation through Computational Fluid Dynamics (CFD) Modeling. Catalysts. 2021; 11(8):1005. https://doi.org/10.3390/catal11081005
Chicago/Turabian StyleAhn, Jeongyoon, Heysuk Kim, Yeonhee Ro, Jintae Kim, Woojin Chung, and Soonwoong Chang. 2021. "Development of Pilot-Scale CO2 Methanation Using Pellet-Type Catalysts for CO2 Recycling in Sewage Treatment Plants and Its Validation through Computational Fluid Dynamics (CFD) Modeling" Catalysts 11, no. 8: 1005. https://doi.org/10.3390/catal11081005