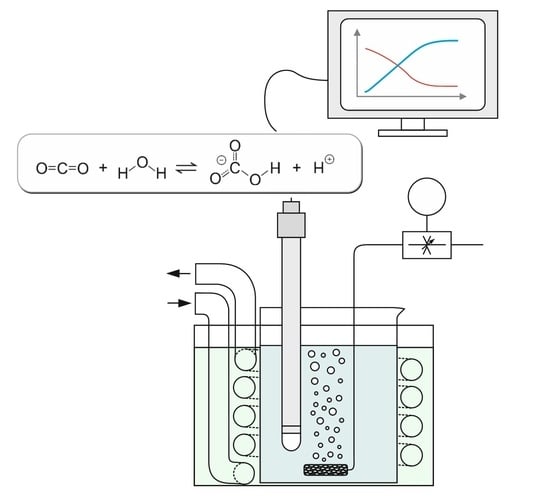
The presented assay is a modification of the Wilbur-Anderson protocol and the only analytical instrument employed is a pH meter with data recording. In order to allow adequate monitoring at higher temperatures by considering the associated enhanced enzyme activities, the time span necessary for a certain pH drop needs to be extended. For this purpose, the buffer concentration of the solution was raised from 20 to 100 mM. The buffer partially adsorbs the protons produced by the enzymatic conversion. In return, more CO2 is also required for the overall reaction. Regarding the limited solubility of CO2, it has to be continuously delivered by means of gas sparging instead of providing a specific amount at the beginning of the test.
The development and evaluation of the method are based on the considerations and assumptions presented in the following. In some equations, amounts (in mol) are addressed whereas concentrations (in mol·L−1) are required for other equations describing equilibrium constants or kinetic reactions. However, taking a hypothetical reaction volume of 1 L allows the one-to-one interconversion of the two units.
2.1. Dependency of Bicarbonate Concentration on pH
The first step was to calculate the relation between measured pH and bicarbonate derived from conversion of CO
2. The apparent pK
a of carbonic acid, 6.35 at 25 °C, indicates a relatively weak acid being only slightly dissociated. However, it is well known that during the aqueous phase carbonic acid is in equilibrium with dissolved or aquatic CO
2 (CO
2(a)). Both species can hardly be distinguished and the above-named value is actually comprised of two equilibria: interconversion of CO
2(a)/H
2CO
3 and the true pK
a. In fact, carbonic acid is a relatively strong acid. The exact pK
a value cannot be determined but it lies between 3.4 and 3.9 at room temperature 20 °C < T < 25 °C and at zero ionic strength [
14]. The pH range observed in the investigated test system, i.e., ≥6.4 is at least 2.5 units higher. Based on the given definition of the pK
a, Equation (1) can be developed.
Equation (1) shows that even at pH 6.4 less than 3.2‰, carbonic acid is present in the protonated form. It can therefore be simplified that the amount of CO
2 dissolved during the aquatic phase that undergoes further conversion corresponds to the HCO
3− ions present in the solution and an equivalent number of released protons. These protons either interact with the TRIS buffer system and/or causes a change of pH.
X is the total protons released that is equivalent to bicarbonate ions [HCO3−].
a denotes the H+ consumed by the buffer components.
b denotes the H+ resulting in a change of pH.
Equation (3) describes the dissociation equilibrium of the TRIS buffer at the initial conditions.
Ka denotes the acid constant of TRIS.
Index ‘i’ indicates initial the concentration values at the beginning of the test.
At a given time, the new equilibrium concentrations are described as follows.
Considering that the proton concentration at the beginning is very low, 10
−8.2, and is almost immediately outweighed by the protons released from H
2CO
3 dissociation, it can be simplified that the absolute proton concentration is equal to b. This simplification is a standard approach also used in pH calculations of weak acids or puffer systems described in the following.
Substituting Equations (4) and (5) into Equation (6) yields the following.
Solving the set of Equations (2) and (7) for [HCO
3−] as a function of [H
+] provides the following.
With pK
a = 8.1 (at 25 °C [
15,
16]) and a total buffer molarity of 0.1, the initial concentrations of the two buffer species at the start of pH (8.2) are the following: [TRIS]
i = 0.056 and [TRISH
+]
i = 0.044. Inserting these values yields Equation (9), which allows the calculation of the bicarbonate concentration dependent on the measured pH as described as follows.
Using pH data, the gained bicarbonate concentration can be plotted versus time.
In order to prove that the resulting simplifications does not produce any significant errors, a more comprehensive calculation was performed. This included two more equations covering the ion dissociation of water and the initial proton concentration, described as follows.
The value, r, denotes the re-association of H+ and OH- to maintain the ion-product of water (=10−14).
Recalculated data almost completely matched the data obtained before. It was therefore concluded that the simpler Equation (9) is sufficiently accurate for the proposed method.
2.2. Correlation between Bicarbonate and Enzyme Reactivity
The second step is the correlation of the bicarbonate concentration to the activity of the CA considering the CO
2 transfer to the aquatic phase. In contrast to the buffer reaction above, the steps discussed in what follows are rate limiting. Consequently, the timely change of concentrations and not the concentrations at equilibrium needs to be addressed. Firstly, the time course of CO
2 in the liquid phase was regarded. The course of gas transfer to liquid is described by the well-known differential Equation (12). Therein, the transfer rate correlates to the difference between the saturation concentration of CO
2 in the liquid phase (C
sat, which depends on the Henry constant) and the actual concentration at a given time multiplied by the mass transfer rate from gas to liquid k
1 [
17]. The latter is specific for a given set-up, which implying parameters such as gas flow rate, bubble size or mixing conditions, etc., while the saturation concentration can be calculated from the Henry coefficient or may otherwise be obtained from respective tables.
Solving this differential equation with a dissolved CO
2 concentration of zero at time zero results in Equation (13).
Csat denotes the saturation concentration of CO2(a).
This function describes the increasing form of an exponential decay and is generally valid for gas transfer reactions, e.g., for oxygenation. However, in the current case it is necessary to include the further reaction of dissolved CO
2 into bicarbonate and a proton. Following the law of mass action, both the forward and the backward reactions have to be taken into account. Therefore, Equation (12) needs to be extended by the following term.
k
2 is the rate constant of CO
2 conversion and k
−2 is the rate constant of the back-reaction in the reverse direction. Combination of Equations (13) and (14) provides the timely change of CO
2 in the liquid phase. However, using Equation (9) to solve the complete differential equation is mathematically complex. Again, a simplification was employed: In the beginning, the back-reaction is very small due to the low concentrations of bicarbonate multiplied by the low concentration of protons. Therefore, the second part of the term can be neglected, which resulting in the following equation.
Rearrangement results in the following equation.
Integration of Equation (16) results in the same type of exponential function as Equation (12), however, with modified parameters.
Equation (17) describes the course of CO
2 concentration as long as the back reaction of bicarbonate is small. With this equation in mind, the use of a CO
2 sensitive electrode is another interesting option for following enzyme activity as it has been investigated by Botrè and Botrè [
18]. However, this alternative was not tested here. In the current case, further development of the relationship between CO
2(a) and HCO
3− concentration was worked out. In Equation (12), this context has already been addressed. Evidently, any CO
2 converted occurs in the form of bicarbonate. Again, disregarding the backreaction due to the distance to the equilibrium concentrations, Equation (18) and, subsequently, Equation (19) can be developed.
In solving the differential equation with the initial condition, the concentration of zero at time zero yields Equation (20).
2.3. Elaboration of the Rate Constant of Enzymatic Turnover
It might be possible to utilize a curve-fitting program and to investigate the individual constants. Nevertheless, an easier solution was sought here. The exponential term in the beginning of Equation (19) is another e-function. With a negative exponent, it gradually progresses towards zero and the HCO
3− concentration becomes a straight line. The slope (m) of the graph is described as follows.
The value of m can be directly taken from the plot of the bicarbonate concentration. Using the blank experiment, k
1 can be elaborated from the rearranged Equation (21) employing literature data for C
sat. and k
2. C
sat is 0.0343 mol.L
−1 at 25 ° C and 1 atm pressure (calculated from the Henry coefficient provided by [
19]). Regarding the second parameter, it must be noted that the actual situation is more complex and that k
2 is not a constant value. The reason is that there are two chemical reactions involved in the CO
2/HCO
3− equilibrium: CO
2 + H
2O ⇔ HCO
3− + H
+ and CO
2 + OH
− ⇔ HCO
3−. According to Schulz et al. [
20] the rate constant at 25 °C can be calculated by combining the two individual reaction constants: k
2 = k
2,1 + k
2,2 × [OH] with k
2,1 = 3.71 × 10
−2·s
−1 and k
2,2 = 2.24 × 10
3 L·mol
−1 s
−1. Despite these explanations, we presume that the assumption of a fixed k
2 is acceptable for the for the current purpose and keeping in mind all the other simplifications already made. In the given pH measurement range (~8–6.5), a k
2 value for the uncatalyzed reaction of 3.8 × 10
−2 is considered a reasonable approximation. According to these provisions, the estimated value for k
1 is 0.051 s
−1 for the specific set-up.
Modeling was employed to crosscheck the general validity of the described methodical approach. Firstly, model parameters were adjusted to fit the data for the blank experiment. Literature values or approximated values as listed in
Table 1 (see
Section 3. Materials and Methods) were applied. In a subsequent step, the CO
2 transfer rate, k
1, was adjusted to best possibly fit the observed data.
Figure 1 shows an overlay of the predicated course and actually measured pH values (a) as well as the predicted and calculated HCO
3− concentration (b) for two experiments with or without the addition of CA, respectively. Despite certain deviations, in particular, for the blank experiment, the model reproduces the data observed for the CA catalyzed reaction and the hydration of CO
2 dominates quite well. It should be noted that the final applied C
sat value was higher than initially considered. The locally higher dynamic pressure at the surface of the sparging device is a probable explanation for that.
An example of model predictions depicting an entire view of all involved chemical species is provided in
Figure 2. Again, the practically linear increase in HCO
3− concentration is demonstrated.
Knowing the slope of the graph and the elaboration of k
1 and C
sat from the blank experiment allows the theoretical calculation of the catalyzed rate constant k
2 in each test. However, it has to be considered that the provision of the exact values for the individual constants is complex. The difficulties related to k
2 were already mentioned. Moreover, C
sat is not only related to temperature and pressure but has also significant dependency on salinity or, more accurately, on the concentration and nature of the individual ions present in aquatic phase [
21]. Even the pK
a of the Tris buffer is only valid in highly diluted solutions and has to be slightly corrected for ionic strength [
22]. Finally, the last simplification made above which disregards the back reaction has some limitations. The back reaction sets in before the bicarbonate concentration reaches its highest inclination and, consequently, the maximum slope is slightly underestimated.
To summarize, there are certain imprecisions regarding the accuracy of the underlying parameters, as discussed previously. Nevertheless, the developed theory allows the reasonable estimation of the k2 of the catalyzed reaction, which permits good judgement of the practical potential of a certain enzyme.
2.4. Calibration with a Standard Enzyme and Overall Assessment
For practical purposes, it was more straightforward and convenient to calibrate the test system with a standard enzyme of known activity. For this purpose, different concentrations of CA from bovine erythrocytes were subjected to the test. The corresponding calculations using pH data and Equation (9) HCO
3− concentrations are provided in
Figure 3a.
Figure 3b demonstrates data evaluation through the determination of the slope of the graph. Even if it might be hard to precisely define the linear range, the inclination within any reasonable time frame of reasonable linearity can be taken as calibration values. The final elaborated calibration curve presenting the applied enzyme amounts (primary x-axis) or the corresponding Wilbur-Andersen units (secondary x-axis) versus the slope of HCO
3− is presented in
Figure 4.
A comparison of the standard enzyme and three further CAs is presented in
Figure 5. Despite an obvious correlation between the two assays, the figure also illustrates that the general assessment of the potential of different enzymes does not provide the same picture. This underlines our hesitations regarding the validity of data obtained by the WA assay conducted at 0–4 °C due to the difference in temperature sensitivity of CAs.
A certain disadvantage of the proposed method is that the pH is not constant throughout the test. Although the buffer provides a certain stabilization effect, pH still drops in the course of the enzyme reaction. Accordingly, enzyme activity is not a distinct value at fixed conditions but an average value over a certain pH range. However, it has to be emphasized that this disadvantage is shared with the other commonly applied test, which is the Wilbur-Anderson assay. It is, of course, possible to combine the presented method with a titration approach to maintain the pH at a fixed set-point. A corresponding attempt has been already suggested by Magid and Turbeck [
23]. However, this would imply an exchange of simplicity for higher accuracy and this would give up the biggest asset of the proposed test system. Despite certain shortcomings and keeping in mind the downsides of other CA assays, we particularly acknowledge the high practicability of the test. In our lab, we found the proposed assay extremely helpful for the purpose of a quick routine method to straightforwardly crosscheck or compare the CO
2 conversion efficiency of different CAs.
Table 1.
Types of reaction, rate laws and constants used for modeling the timely behavior of chemical species.
 H+ + OH-
H+ + OH- H+ + HCO3−
H+ + HCO3− TRIS + H+
TRIS + H+