Ionic Liquid-Assisted Synthesis of Ag3PO4 Spheres for Boosting Photodegradation Activity under Visible Light
Abstract
:1. Introduction
2. Results and Discussion
2.1. XRD Analysis
2.2. SEM Analysis
2.3. DRS Analysis
2.4. XPS Analysis
2.5. Photocatalytic Activity
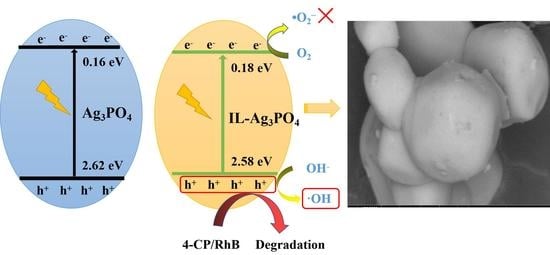
2.6. Photocatalytic Mechanism Study
3. Experimental Section
3.1. Materials
3.2. Ionic Liquid-Assisted Synthesis of Ag3PO4 Spheres
3.3. Characterization of Photocatalysts
3.4. Photocatalytic Activity
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wu, K.; Li, F.R.; Guo, J.; Zou, J.; Li, H.; Li, J.D.; Wu, H.D.; Zhang, L.F. Synthesis of Heterostructure g-C3N4/h-MoO3 with Enhanced Photoactivity: Influencing Factors and Mechanism Insight. Nano 2021, 16, 2150024. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, Q.F.; Hou, W.H.; Zou, Z.G. 2D Titanium/Niobium Metal Oxide-Based Materials for Photocatalytic Application. Sol. RRL 2020, 4, 2000070. [Google Scholar] [CrossRef]
- Bianca, R.; Salvador, E.; de Hugo, L. Hydrogen Production via Pd-TiO2 Photocatalytic Water Splitting under Near-UV and Visible Light: Analysis of the Reaction Mechanism. Catalysts 2021, 11, 405. [Google Scholar] [CrossRef]
- Wang, W.; Fang, J.J.; Shao, S.F.; Lai, M.; Lu, C.H. Compact and uniform TiO2@g-C3N4 core-shell quantum heterojunction for photocatalytic degradation of tetracycline antibiotics. Appl. Catal. B Environ. 2017, 217, 57–64. [Google Scholar] [CrossRef]
- Luo, L.; Li, Y.Z.; Hou, J.T.; Yang, Y. Visible photocatalysis and photostability of Ag3PO4 photocatalyst. Appl. Surf. Sci. 2014, 319, 332–338. [Google Scholar] [CrossRef]
- Mo, Z.; Xu, H.; Chen, Z.G.; She, X.J.; Song, Y.H.; Wu, J.J.; Yan, P.C.; Xu, L.; Lei, Y.C.; Yuan, S.Q.; et al. Self-assembled synthesis of defect-engineered graphitic carbon nitride nanotubes for efficient conversion of solar energy. Appl. Catal. B Environ. 2018, 225, 154–161. [Google Scholar] [CrossRef]
- Lai, M.T.L.; Lai, C.W.; Lee, K.M.; Chook, S.W.; Yang, T.C.K.; Chong, S.H.; Juan, J.C. Facile one-pot solvothermal method to synthesize solar active Bi2WO6 for photocatalytic degradation of organic dye. J. Alloys Compd. 2019, 801, 502–510. [Google Scholar] [CrossRef]
- Sarkar, S.; Chattopadhyay, K.K. Visible light photocatalysis and electron emission from porous hollow spherical BiVO4 nanostructures synthesized by a novel route. Phys. E. 2014, 58, 52–58. [Google Scholar] [CrossRef]
- Yi, Z.G.; Ye, J.H.; Kikugawa, N.; Kako, T.; Ouyang, S.X.; Stuart-Williams, H.; Yang, H.; Cao, J.Y.; Luo, W.J.; Li, Z.S.; et al. An orthophosphate semiconductor with photooxidation properties under visible-light irradiation. Nat. Mater. 2010, 9, 559–564. [Google Scholar] [CrossRef]
- Ge, M.; Li, Z.L. Recent progress in Ag3PO4-based all-solid-state Z-scheme photocatalytic systems. Chin. J. Catal. 2017, 38, 1794–1803. [Google Scholar] [CrossRef]
- Li, X.P.; Xu, P.; Chen, M.; Zeng, G.M.; Wang, D.B.; Chen, F.; Tang, W.W.; Chen, C.Y.; Zhang, C.; Tan, X.F. Application of silver phosphate-based photocatalysts: Barriers and solutions. Chem. Eng. J. 2019, 366, 339–357. [Google Scholar] [CrossRef]
- Xie, Y.; Huang, Z.H.; Zhang, Z.J.; Zhang, X.G.; Wen, R.L.; Liu, Y.G.; Fang, M.H.; Wu, X.W. Controlled synthesis and photocatalytic properties of rhombic dodecahedral Ag3PO4 with high surface energy. Appl. Surf. Sci. 2016, 389, 56–66. [Google Scholar] [CrossRef]
- Dong, P.Y.; Yin, Y.; Xu, N.; Guan, R.F.; Hou, G.H.; Wang, Y.H. Facile synthesis of tetrahedral Ag3PO4 mesocrystals and its enhanced photocatalytic activity. Mater. Res. Bull. 2014, 60, 682–689. [Google Scholar] [CrossRef]
- Zhang, Y.; Duoerkun, G.; Shi, Z.; Cao, W.; Liu, T.; Liu, J.S.; Zhang, L.S.; Li, M.Q.; Chen, Z.G. Construction of TiO2/Ag3PO4 nanojunctions on carbon fiber cloth for photocatalytically removing various organic pollutants in static or flowing wastewater. J. Colloid Interface Sci. 2020, 571, 213–221. [Google Scholar] [CrossRef]
- Martin, D.J.; Umezawa, N.; Chen, X.W.; Ye, J.H.; Tang, J.W. Facet engineered Ag3PO4 for efficient water photooxidation†. Energy Environ. Sci. 2013, 6, 3380–3386. [Google Scholar] [CrossRef]
- He, S.X.; Zhai, C.Y.; Fujitsuka, M.; Kim, S.; Zhu, M.S.; Yin, R.L.; Zeng, L.X.; Majima, T. Femtosecond time-resolved diffuse reflectance study on facet engineered charge-carrier dynamics in Ag3PO4 for antibiotics photodegradation. Appl. Catal. B Environ. 2021, 281, 119479–119488. [Google Scholar] [CrossRef]
- Bhatt, D.K.; Patel, U.D. Photocatalytic degradation of Reactive Black 5 using Ag3PO4 under visible light. J. Phys. Chem. Solids 2021, 149, 109768–109778. [Google Scholar] [CrossRef]
- Hsieh, M.S.; Su, H.J.; Hsieh, P.L.; Chiang, Y.W.; Huang, M.H. Synthesis of Ag3PO4 Crystals with Tunable Shapes for Facet-Dependent Optical Property, Photocatalytic Activity, and Electrical Conductivity Examinations. ACS. Appl. Mater. Interfaces 2017, 9, 39086–39093. [Google Scholar] [CrossRef]
- Bi, Y.P.; Hu, H.Y.; Jiao, Z.B.; Yu, H.C.; Lu, G.X.; Ye, J.H. Two-dimensional dendritic Ag3PO4 nanostructures and their photocatalytic properties. Phys. Chem. Chem. Phys. 2012, 14, 14486–14488. [Google Scholar] [CrossRef]
- Pongsaton, A.; Khanitta, I.; Sumetha, S.; Jonas, B. Effect of Phosphate Salts (Na3PO4, Na2HPO4, and NaH2PO4) on Ag3PO4 Morphology for Photocatalytic Dye Degradation under Visible Light and Toxicity of the Degraded Dye Products. Ind. Eng. Chem. Res. 2013, 52, 17369–17375. [Google Scholar] [CrossRef]
- Chen, F.; Huang, S.Q.; Xu, Y.G.; Huang, L.Y.; Wei, W.; Xu, H.; Li, H.M. Novel ionic liquid modified carbon nitride fabricated by in situ pyrolysis of 1-butyl-3-methylimidazolium cyanamide to improve electronic structure for efficiently degradation of bisphenol A. Colloids. Surf. A 2021, 610, 125648. [Google Scholar] [CrossRef]
- Di, J.; Xia, J.X.; Ge, Y.P.; Xu, L.; Xu, H.; He, M.Q.; Zhang, Q.; Li, H.M. Reactable ionic liquid-assisted rapid synthesis of BiOI hollow microspheres at room temperature with enhanced photocatalytic activity. J. Mater. Chem. A 2014, 2, 15864–15874. [Google Scholar] [CrossRef]
- Wang, B.; Di, J.; Zhang, P.F.; Xia, J.X.; Dai, S.; Li, H.M. Ionic liquid-induced strategy for porous perovskite-like PbBiO2Br photocatalysts with enhanced photocatalytic activity and mechanism insight. Appl. Catal. B Environ. 2017, 206, 127–135. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.G.; Zeng, J.; Di, J.; Zhao, D.X.; Ji, M.X.; Xia, J.X.; Li, H.M. Facile microwave-assisted ionic liquid synthesis of sphere-like BiOBr hollow and porous nanostructures with enhanced photocatalytic performance. Green Energy Environ. 2017, 2, 124–133. [Google Scholar] [CrossRef]
- Li, F.F.; Li, Z.H.; Zhang, M.X.; Shen, Y.; Cai, Y.F.; Li, Y.R.; He, X.Y.; Chen, C. Ag3PO4@holmium phosphate core@shell composites with enhanced photocatalytic activity. RSC Adv. 2017, 7, 34705–34713. [Google Scholar] [CrossRef] [Green Version]
- Alammar, T.; Smetana, V.; Pei, H.; Hamm, I.; Wark, M.; Mudring, A.V. The Power of Ionic Liquids: Crystal Facet Engineering of SrTiO3 Nanoparticles for Tailored Photocatalytic Applications. Adv. Sustain. Syst. 2020, 5, 2000180–2000189. [Google Scholar] [CrossRef]
- Amir Mohammad Sheikh, A.; Mohammad, M.; Elaheh, K.; Hossein, A. Ionic liquid-assisted sol-gel synthesis of Fe2O3-TiO2 for enhanced photocatalytic degradation of bisphenol a under UV illumination: Modeling and optimization using response surface methodology. Optik 2020, 204, 164229–164240. [Google Scholar] [CrossRef]
- Chen, J.; Xia, J.X.; Di, J.; Ji, M.X.; Li, H.P.; Xu, H.; Zhang, Q.; Lu, J. Reactable ionic liquid assisted synthesis of BiPO4 and the influences of solvent on structure, morphology and photocatalytic performance. Colloids Surf. A 2016, 488, 110–117. [Google Scholar] [CrossRef]
- Xia, J.X.; Ji, M.X.; Di, J.; Wang, B.; Yin, S.; Zhang, Q.; He, M.Q.; Li, H.M. Construction of ultrathin C3N4/Bi4O5I2 layered nanojunctions via ionic liquid with enhanced photocatalytic performance and mechanism insight. Appl. Catal. B Environ. 2016, 191, 235–245. [Google Scholar] [CrossRef]
- Liu, C.; Sun, T.; Wu, L.; Liang, J.Y.; Huang, Q.J.; Chen, J.; Hou, W.H. N-doped Na2Ti6O13@TiO2 core–shell nanobelts with exposed {1 0 1} anatase facets and enhanced visible light photocatalytic performance. Appl. Catal. B Environ. 2015, 170, 17–24. [Google Scholar] [CrossRef]
- Li, J.Q.; Yuan, H.; Zhu, Z.F. In situ growth of Ag3PO4 on N-BiPO4 nanorod: A core-shell heterostructure for high performance photocatalyst. J. Colloid Interface Sci. 2016, 462, 382–388. [Google Scholar] [CrossRef]
- Zhang, H.; Tang, G.G.; Wan, X.; Xu, J.; Tang, H. High-efficiency all-solid-state Z-scheme Ag3PO4/g-C3N4/ MoSe2 photocatalyst with boosted visible-light photocatalytic performance for antibiotic elimination. Appl. Surf. Sci. 2020, 530, 147234–147243. [Google Scholar] [CrossRef]
- Sun, M.; Zeng, Q.; Zhao, X.; Shao, Y.; Ji, P.G.; Wang, C.Q.; Yan, T.; Du, B. Fabrication of novel g-C3N4 nanocrystals decorated Ag3PO4 hybrids: Enhanced charge separation and excellent visible-light driven photocatalytic activity. J. Hazard. Mater. 2017, 339, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.Y.; Hu, F.X.; Chen, W.W.; Han, Z.T.; Liu, C.; Dai, Y. ZnCuAl-LDH/Bi2MoO6 Nanocomposites with Improved Visible Light-Driven Photocatalytic Degradation. Acta. Phys. Chim. Sin. 2020, 36, 1911016. [Google Scholar] [CrossRef]
- Liu, C.; Zhu, H.J.; Zhu, Y.S.; Dong, P.Y.; Hou, H.J.; Xu, Q.X.; Chen, X.W.; Xi, X.G.; Hou, W.H. Ordered layered N-doped KTiNbO5/g-C3N4 heterojunction with enhanced visible light photocatalytic activity. Appl. Catal. B Environ. 2018, 228, 54–63. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, X.W.; Zhang, Y.; Zhu, J.Y.; Zhu, R.L. Nano flake Ag3PO4 enhanced photocatalytic activity of bisphenol A under visible light irradiation. Colloid Interface Sci. Commun. 2020, 37, 100277–100284. [Google Scholar] [CrossRef]
- Liu, C.; Xu, Q.X.; Zhang, Q.F.; Zhu, Y.S.; Ji, M.W.; Tong, Z.W.; Hou, W.H.; Zhang, Y.; Xu, J.G. Layered BiOBr/Ti3C2 MXene composite with improved visible-light photocatalytic activity. J. Mater. Sci. 2018, 54, 2458–2471. [Google Scholar] [CrossRef]
- Liu, C.; Feng, Y.; Han, Z.T.; Sun, Y.; Wang, X.Q.; Zhang, Q.F.; Zou, Z.G. Z-scheme N-doped K4Nb6O17/g-C3N4 heterojunction with superior visible-light-driven photocatalytic activity for organic pollutant removal and hydrogen production. Chin. J. Catal. 2020, 42, 164–174. [Google Scholar] [CrossRef]
- Liu, C.; Han, Z.T.; Feng, Y.; Dai, H.L.; Zhao, Y.F.; Han, N.; Zhang, Q.F.; Zou, Z.G. Ultrathin Z-scheme 2D/2D N-doped HTiNbO5 nanosheets/g-C3N4 porous composites for efficient photocatalytic degradation and H2 generation under visible light. J. Colloid Interface Sci. 2020, 583, 58–70. [Google Scholar] [CrossRef]
- Liu, C.; Wu, Q.S.; Ji, M.W.; Zhu, H.J.; Hou, H.J.; Yang, Q.H.; Jiang, C.F.; Wang, J.J.; Tian, L.; Chen, J.; et al. Constructing Z-scheme charge separation in 2D layered porous BiOBr/graphitic C3N4 nanosheets nanojunction with enhanced photocatalytic activity. J. Alloys Compd. 2017, 723, 1121–1131. [Google Scholar] [CrossRef]
- Ji, M.X.; Di, J.; Liu, Y.L.; Chen, R.; Li, K.; Chen, Z.G.; Xia, J.X.; Li, H.M. Confined active species and effective charge separation in Bi4O5I2 ultrathin hollow nanotube with increased photocatalytic activity. Appl. Catal. B Environ. 2020, 268, 118403–118413. [Google Scholar] [CrossRef]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, B.; Zhang, L.; Zhang, Y.; Liu, C.; Xia, J.; Li, H. Ionic Liquid-Assisted Synthesis of Ag3PO4 Spheres for Boosting Photodegradation Activity under Visible Light. Catalysts 2021, 11, 788. https://doi.org/10.3390/catal11070788
Zhang B, Zhang L, Zhang Y, Liu C, Xia J, Li H. Ionic Liquid-Assisted Synthesis of Ag3PO4 Spheres for Boosting Photodegradation Activity under Visible Light. Catalysts. 2021; 11(7):788. https://doi.org/10.3390/catal11070788
Chicago/Turabian StyleZhang, Beibei, Lu Zhang, Yulong Zhang, Chao Liu, Jiexiang Xia, and Huaming Li. 2021. "Ionic Liquid-Assisted Synthesis of Ag3PO4 Spheres for Boosting Photodegradation Activity under Visible Light" Catalysts 11, no. 7: 788. https://doi.org/10.3390/catal11070788