Immobilized Alcalase on Micron- and Submicron-Sized Alginate Beads as a Potential Biocatalyst for Hydrolysis of Food Proteins
Abstract
:1. Introduction
2. Results and Discussion
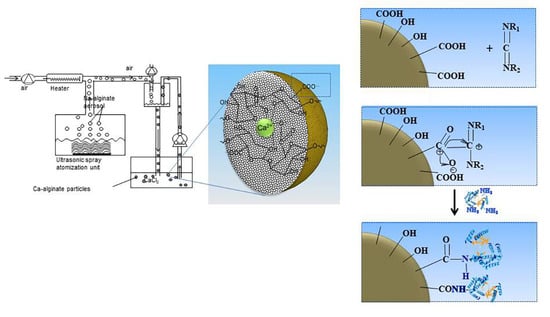
2.1. Covalent Immobilization of Alcalase
2.2. Beads Characterization
2.3. FT-IR Analysis
2.4. Optimum Conditions for Alcalase Immobilization
2.5. Application of the Immobilized Alcalase in the Industrially Feasible Reaction System of Food Proteins Hydrolysis
3. Materials and Methods
3.1. Materials
3.2. Preparation of Calcium Alginate Beads by Electrostatic Extrusion Technique
3.3. Preparation of Calcium Alginate Beads by Ultrasonic Spray Atomization System
3.4. Immobilization Method
3.5. Alcalase Activity Assay
3.6. FT-IR Analysis
3.7. Bead Size
3.8. Scanning Electron Microscopy (SEM)
3.9. Enzymatic Hydrolysis of Egg White Protein and Soy Protein Isolate with the Immobilized Alcalase
3.10. Determining of Degree of Hydrolysis
3.11. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Meinlschmidt, P.; Sussmann, D.; Schweiggert-Weisz, U.; Eisner, P. Enzymatic treatment of soy protein isolates: Effects on the potential allergenicity, technofunctionality, and sensory properties. Food Sci. Nutr. 2016, 4, 11–23. [Google Scholar] [CrossRef]
- Kılıç Apar, D.; Özbek, B. Corn gluten hydrolysis by alcalase: Effects of process parameters on hydrolysis, solubilization and enzyme inactivation. Chem. Biochem. Eng. Q. 2008, 22, 203–212. [Google Scholar]
- Anh, T.L.Q.; Hoa, N.T.Q.; Nguyen, P.D.T.; Thanh, H.V.; Nguyen, P.B.; Anh, L.T.H.; Dao, D.T.A. Soybean protein extraction by alcalase and flavourzyme, combining thermal pretreatment for enteral feeding product. Catalysts 2020, 10, 829. [Google Scholar] [CrossRef]
- Stefanović, A.B.; Jovanović, J.R.; Grbavčić, S.; Šekuljica, N.; Manojlović, V.B.; Bugarski, B.M.; Knežević-Jugović, Z.D. Impact of ultrasound on egg white proteins as a pretreatment for functional hydrolysates production. Eur. Food Res. Technol. 2014, 239, 979–993. [Google Scholar] [CrossRef]
- Liu, D.M.; Chen, J.; Shi, Y.P. Advances on methods and easy separated support materials for enzymes immobilization. Trends Analyt. Chem. 2018, 102, 332–342. [Google Scholar] [CrossRef]
- Sewczyk, T.; Hoog Antink, M.; Maas, M.; Kroll, S.; Beutel, S. Flow rate dependent continuous hydrolysis of protein isolates. AMB Express 2018, 8, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tavano, O.L.; Berenguer-Murcia, A.; Secundo, F.; Fernandez-Lafuente, R. Biotechnological applications of proteases in food technology. Compr. Rev. Food Sci. Food Saf. 2018, 17, 412–436. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Galan, C.; Berenguer-Murcia, Á.; Fernandez-Lafuente, R.; Rodrigues, R.C. Potential of different enzyme immobilization strategies to improve enzyme performance. Adv. Synth. Catal. 2011, 353, 2885–2904. [Google Scholar] [CrossRef]
- Mateo, C.; Grazu, V.; Palomo, J.M.; Lopez-Gallego, F.; Fernandez-Lafuente, R.; Guisan, J.M. Immobilization of enzymes on heterofunctional epoxy supports. Nat. Protoc. 2007, 2, 1022–1033. [Google Scholar] [CrossRef] [PubMed]
- Knežević, Z.D.; Bobić, S.; Milutinović, A.; Obradović, B.; Mojović, L.; Bugarski, B. Alginate-immobilized lipase by electrostatic extrusion for the purpose of palm oil hydrolysis in lecithin/isooctane system. Process Biochem. 2002, 38, 313–318. [Google Scholar] [CrossRef]
- Kumar, S.; Haq, I.; Prakash, J.; Raj, A. Improved enzyme properties upon glutaraldehyde cross-linking of alginate entrapped xylanase from Bacillus licheniformis. Int. J. Biol. Macromol. 2017, 98, 24–33. [Google Scholar] [CrossRef]
- Corstens, M.N.; Berton-Carabin, C.C.; Schroën, K.; Viau, M.; Meynier, A. Emulsion encapsulation in calcium-alginate beads delays lipolysis during dynamic in vitro digestion. J. Funct. Foods 2018, 46, 394–402. [Google Scholar] [CrossRef]
- Lević, S.; Pajić Lijaković, I.; Đorđević, V.; Rac, V.; Rakić, V.; Šolević Knudsen, T.; Pavlović, V.; Bugarski, B.; Nedović, V. Characterization of sodium alginate/d-limonene emulsions and respective calcium alginate/D-limonene beads produced by electrostatic extrusion. Food Hydrocoll. 2015, 45, 111–123. [Google Scholar] [CrossRef] [Green Version]
- De Carvalho Silva, J.; França, P.R.L.; Converti, A.; Porto, T.S. Kinetic and thermodynamic characterization of a novel Aspergillus aculeatus URM4953 polygalacturonase. Comparison of free and calcium alginate-immobilized enzyme. Process Biochem. 2018, 74, 61–70. [Google Scholar] [CrossRef]
- Jia, L.; Wong, H.; Cerna, C.; Weitman, S.D. Effect of nanonization on absorption of 301029: Ex vivo and in vivo pharmacokinetic correlations determined by liquid chromatography/mass spectrometry. Pharm. Res. 2002, 19, 1091–1096. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, A.S.S.; Al-Salamah, A.A.; El-Toni, A.M.; Almaary, K.S.; El-Tayeb, M.A.; Elbadawi, Y.B.; Antranikian, G. Enhancement of alkaline protease activity and stability via covalent immobilization onto hollow core-mesoporous shell silica nanospheres. Int. J. Mol. Sci. 2016, 17, 184. [Google Scholar] [CrossRef] [Green Version]
- Zeng, Q.; Li, Q.; Sun, D.; Zheng, M. Alcalase microarray base on metal ion modified hollow mesoporous silica spheres as a sustainable and efficient catalysis platform for proteolysis. Front. Bioeng. Biotechnol. 2020, 8, 565. [Google Scholar] [CrossRef]
- Mora-Huertas, C.E.; Fessi, H.; Elaissari, A. Polymer-based nanocapsules for drug delivery. Int. J. Pharm. 2010, 385, 113–142. [Google Scholar] [CrossRef]
- Sonavane, G.S.; Devarajan, P.V. Preparation of alginate nanoparticles using Eudragit E100 as a new complexing agent: Development, in-vitro, and in-vivo evaluation. J. Biomed. Nanotechnol. 2007, 3, 160–169. [Google Scholar] [CrossRef]
- Yeo, Y.; Park, K. A new microencapsulation method using an ultrasonic atomizer based on interfacial solvent exchange. J. Control. Release 2004, 100, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Dalmoro, A.; d’Amore, M.; Barba, A.A. Droplet size prediction in the production of drug delivery microsystems by ultrasonic atomization. Transl. Med. UniSa 2013, 7, 6–11. [Google Scholar] [PubMed]
- Prasad, R.; Jayakumaran Nair, A. Immobilization of Bacillus sp. Protease on different matrices and its enzymatic characterization. Int. J. Pharma Bio Sci. 2013, 4, 2013. [Google Scholar]
- Bedzo, O.K.K.; Trollope, K.; Gottumukkala, L.D.; Coetzee, G.; Görgens, J.F. Amberlite IRA 900 versus calcium alginate in immobilization of a novel, engineered β-fructofuranosidase for short-chain fructooligosaccharide synthesis from sucrose. Biotechnol. Prog. 2019, 35, 1–9. [Google Scholar] [CrossRef]
- Domínguez, E.; Nilsson, M.; Hahn-Hägerdal, B. Carbodiimide Coupling of β-galactosidase from Aspergillus oryzae to alginate. Enzyme Microb. Technol. 1988, 10, 606–610. [Google Scholar] [CrossRef]
- Tümtürk, H.; Şahin, F.; Demirel, G. A new method for immobilization of acetylcholinesterase. Bioprocess Biosyst. Eng. 2007, 30, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Tee, B.L.; Kaletunç, G. Immobilization of a thermostable α-amylase by covalent binding to an alginate matrix increases high temperature usability. Biotechnol. Prog. 2009, 25, 436–445. [Google Scholar] [CrossRef]
- Lopes, L.A.; Novelli, P.K.; Fernandez-Lafuente, R.; Tardioli, P.W.; Giordano, R.L.C. Glyoxyl-activated agarose as support for covalently link Novo-Pro D: Biocatalysts performance in the hydrolysis of casein. Catalysts 2020, 10, 466. [Google Scholar] [CrossRef]
- Larosa, C.; Salerno, M.; de Lima, J.S.; Merijs Meri, R.; da Silva, M.F.; de Carvalho, L.B.; Converti, A. Characterisation of bare and tannase-loaded calcium alginate beads by microscopic, thermogravimetric, FTIR and XRD analyses. Int. J. Biol. Macromol. 2018, 115, 900–906. [Google Scholar] [CrossRef] [PubMed]
- Davidovich-Pinhas, M.; Harari, O.; Bianco-Peled, H. Evaluating the mucoadhesive properties of drug delivery systems based on hydrated thiolated alginate. J. Control. Release 2009, 136, 38–44. [Google Scholar] [CrossRef]
- Pessato, T.B.; de Carvalho, N.C.; Tavano, O.L.; Fernandes, L.G.R.; Zollner, R.L.; Netto, F.M. Whey protein isolate hydrolysates obtained with free and immobilized alcalase: Characterization and detection of residual allergens. Food Res. Int. 2016, 83, 112–120. [Google Scholar] [CrossRef]
- Corîci, L.N.; Frissen, A.E.; Van Zoelen, D.-J.; Eggen, I.F.; Peter, F.; Davidescu, C.M.; Boeriu, C.G. Synthesis of peptide amides using sol-gel immobilized alcalase in batch and continuous reaction system. World Acad. Sci. Eng. Technol. 2011, 76, 361–366. [Google Scholar]
- Ferreira, L.; Ramos, M.A.; Dordick, J.S.; Gil, M.H. Influence of different silica derivatives in the immobilization and stabilization of a Bacillus licheniformis protease (Subtilisin Carlsberg). J. Mol. Catal. B Enzym. 2003, 21, 189–199. [Google Scholar] [CrossRef] [Green Version]
- Žuža, M.G.; Milašinović, N.Z.; Jonović, M.M.; Jovanović, J.R.; Kalagasidis Krušić, M.T.; Bugarski, B.M.; Knežević-Jugović, Z.D. Design and characterization of alcalase–chitosan conjugates as potential biocatalysts. Bioprocess Biosyst. Eng. 2017, 40, 1713–1723. [Google Scholar] [CrossRef]
- Xie, W.; Ma, N. Enzymatic transesterification of soybean oil by using immobilized lipase on magnetic nano-particles. Biomass Bioenergy 2010, 34, 890–896. [Google Scholar] [CrossRef]
- Das, A.; Singh, J.; Yogalakshmi, K.N. Laccase immobilized magnetic iron nanoparticles: Fabrication and its performance evaluation in chlorpyrifos degradation. Int. Biodeterior. Biodegrad. 2017, 117, 183–189. [Google Scholar] [CrossRef]
- Myrnes, E.E. Alginate Gels Cross-Linked with Mixtures of Calcium and Chitosan Oligomers: Effect on Swelling Properties and Leakage from the Gel Erlend Eikeland Myrnes. Master’s Thesis, Fakultet for Naturvitenskap, Norwegian University of Science and Technology (NTNU), Trondheim, Norway, May 2016. [Google Scholar]
- Nawaz, M.A.; Rehman, H.U.; Bibi, Z.; Aman, A.; Ul Qader, S.A. Continuous degradation of maltose by enzyme entrapment technology using calcium alginate beads as a matrix. Biochem. Biophys. Rep. 2015, 4, 250–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thu, T.T.M.; Krasaekoopt, W. Encapsulation of protease from Aspergillus oryzae and lipase from Thermomyces lanuginoseus using alginate and different copolymer types. Agric. Nat. Resour. 2016, 50, 155–161. [Google Scholar] [CrossRef] [Green Version]
- Martín, M.C.; López, O.V.; Ciolino, A.E.; Morata, V.I.; Villar, M.A.; Ninago, M.D. Immobilization of enological pectinase in calcium alginate hydrogels: A potential biocatalyst for winemaking. Biocatal. Agric. Biotechnol. 2019, 18, 101091. [Google Scholar] [CrossRef] [Green Version]
- Jovanović, J.; Stefanović, A.; Culetu, A.; Duta, D.; Luković, N.; Jakovetić Tanasković, S.; Šekuljica, N.; Knežević-Jugović, Z. Enzymatic treatment of soy protein concentrate: Influence on the potential techno-functional and antioxidant properties. J. Hyg. Eng. Des. 2019, 30, 58–68. [Google Scholar]
- Liu, L.; Li, S.; Zheng, J.; Bu, T.; He, G.; Wu, J. Safety considerations on food protein-derived bioactive peptides. Trends Food Sci. Technol. 2020, 96, 199–207. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Beynon, R.J.; Bond, J.S. Proteolytic Enzymes. A Practical Approach; IRL Press at Oxford University Press: New York, NY, USA, 2001. [Google Scholar]
- Sáez-Plaza, P.; Michałowski, T.; Navas, M.J.; Asuero, A.G.; Wybraniec, S. An overview of the Kjeldahl method of nitrogen determination. Part I. Early history, chemistry of the procedure, and titrimetric finish. Crit. Rev. Anal. Chem. 2013, 43, 178–223. [Google Scholar] [CrossRef]
- Adler-Nissen, J. Determination of the degree of hydrolysis of food protein hydrolysates by trinitrobenzenesulfonic acid. J. Agric. Food Chem. 1979, 27, 1256–1262. [Google Scholar] [CrossRef] [PubMed]








| Title 1 | Title 2 | Zeta Potential (ZP) |
|---|---|---|
| EE beads | 698 ± 0.21 | −6.34 ± 0.33 mV |
| UA beads | 0.428 ± 0.078 | −6.15 ± 0.26 mV |
| UAD beads | 0.261 ± 0.033 | −6.17 ± 0.27 mV |
| alcalase immobilized on EE beads | 803 ± 23 | −28.6 ± 1.40 mV |
| alcalase immobilized on UA beads | 0.607 ± 0.103 | −27.8 ± 1.69 mV |
| alcalase immobilized on UAD beads | 0.394 ± 0.051 | −30.3 ± 1.11 mV |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jonović, M.; Žuža, M.; Đorđević, V.; Šekuljica, N.; Milivojević, M.; Jugović, B.; Bugarski, B.; Knežević-Jugović, Z. Immobilized Alcalase on Micron- and Submicron-Sized Alginate Beads as a Potential Biocatalyst for Hydrolysis of Food Proteins. Catalysts 2021, 11, 305. https://doi.org/10.3390/catal11030305
Jonović M, Žuža M, Đorđević V, Šekuljica N, Milivojević M, Jugović B, Bugarski B, Knežević-Jugović Z. Immobilized Alcalase on Micron- and Submicron-Sized Alginate Beads as a Potential Biocatalyst for Hydrolysis of Food Proteins. Catalysts. 2021; 11(3):305. https://doi.org/10.3390/catal11030305
Chicago/Turabian StyleJonović, Marko, Milena Žuža, Verica Đorđević, Nataša Šekuljica, Milan Milivojević, Branimir Jugović, Branko Bugarski, and Zorica Knežević-Jugović. 2021. "Immobilized Alcalase on Micron- and Submicron-Sized Alginate Beads as a Potential Biocatalyst for Hydrolysis of Food Proteins" Catalysts 11, no. 3: 305. https://doi.org/10.3390/catal11030305