Photocatalytic Porous Silica-Based Granular Media for Organic Pollutant Degradation in Industrial Waste-Streams
Abstract
:1. Introduction
2. Results and Discussion
2.1. Photocatalytic Porous Silica-Based Granular Media Characterization
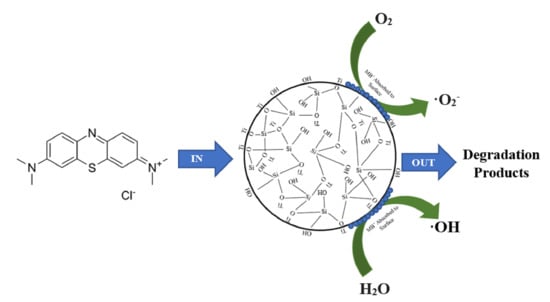
2.2. Photocatalytic Degradation of Methylene Blue
2.2.1. Degradation Kinetics
2.2.2. Effect of pH on Degradation Kinetics
2.2.3. Sustained Photocatalytic Reactivity with Added Amendments to the SGM
2.2.4. Determination of the Most Efficient SGM
2.2.5. SGM Condition after Cyclic Testing
3. Experimental Methods and Materials
3.1. Materials
3.2. Photocatalytic Porous Silica-Based Granular Media Development
3.3. Photocatalytic Porous Silica-Based Granular Media Synthesis
3.4. Photocatalytic Testing
4. Applications and Future Testing
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lellis, B.; Fávaro-Polonio, C.Z.; Pamphile, J.A.; Polonio, J.C. Effects of textile dyes on health and the environment and bioremediation potential of living organisms. Biotechnol. Res. Innov. 2019, 3, 275–290. [Google Scholar] [CrossRef]
- Brillas, E.; Martínez-Huitle, C.A. Decontamination of wastewaters containing synthetic organic dyes by electrochemical methods. An updated review. Appl. Catal. B Environ. 2015, 166–167, 603–643. [Google Scholar] [CrossRef]
- Hassaan, M.A.; El Nemr, A. Health and Environmental Impacts of Dyes: Mini Review. Am. J. Environ. Sci. Eng. 2017, 1, 64–67. [Google Scholar] [CrossRef]
- Raval, N.P.; Shah, P.U.; Shah, N.K. Malachite green “a cationic dye” and its removal from aqueous solution by adsorption. Appl. Water Sci. 2017, 7, 3407–3445. [Google Scholar] [CrossRef] [Green Version]
- Khan, S.; Malik, A. Toxicity evaluation of textile effluents and role of native soil bacterium in biodegradation of a textile dye. Environ. Sci. Pollut. Res. 2018, 25, 4446–4458. [Google Scholar] [CrossRef]
- Hueber-Becker, F.; Nohynek, G.J.; Dufour, E.K.; Meuling, W.J.; De Bie, A.T.; Toutain, H.; Bolt, H.M. Occupational exposure of hairdressers to [14C]-para-phenylenediamine-containing oxidative hair dyes: A mass balance study. Food Chem. Toxicol. 2007, 45, 160–169. [Google Scholar] [CrossRef]
- Cassano, A.; Molinari, R.; Romano, M.; Drioli, E. Treatment of aqueous effluents of the leather industry by membrane processes. J. Membr. Sci. 2001, 181, 111–126. [Google Scholar] [CrossRef]
- Albano, G.; Colli, T.; Nucci, L.; Charaf, R.; Biver, T.; Pucci, A.; Aronica, L.A. Synthesis of new bis[1-(thiophenyl)propynones] as potential organic dyes for colorless luminescent solar concentrators (LSCs). Dyes Pigment. 2020, 174, 108100. [Google Scholar] [CrossRef] [Green Version]
- Samchetshabam, G.; Hussan, A.; Choudhury, T.G. Impact of Textile Dyes Waste on Aquatic Environments and its Treatment Impact of Textile Dyes Waste on Aquatic Environments and its Treatment. Environ. Ecol. 2017, 35, 2349–2353. [Google Scholar]
- Chiu, Y.-H.; Chang, T.-F.M.; Chen, C.-Y.; Sone, M.; Hsu, Y.-J. Mechanistic Insights into Photodegradation of Organic Dyes Using Heterostructure Photocatalysts. Catalysts 2019, 9, 430. [Google Scholar] [CrossRef] [Green Version]
- Katafias, A.; Lipińska, M.; Strutyński, K. Alkaline hydrogen peroxide as a degradation agent of methylene blue—Kinetic and mechanistic studies. React. Kinet. Mech. Catal. 2010, 101, 251–266. [Google Scholar] [CrossRef]
- Aa, O.; Aj, O. Kinetic Study of Decolorization of Methylene Blue with Sodium Sulphite in Aqueous Media: Influence of Transition Metal Ions. J. Phys. Chem. Biophys. 2014, 2, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, M.; Abou-Gamra, Z.; Salem, A. Photocatalytic degradation of methylene blue dye over novel spherical mesoporous Cr2O3/TiO2 nanoparticles prepared by sol-gel using octadecylamine template. J. Environ. Chem. Eng. 2017, 5, 4251–4261. [Google Scholar] [CrossRef]
- Liu, H.; Guo, W.; Li, Y.; He, S.; He, C. Photocatalytic degradation of sixteen organic dyes by TiO2/WO3-coated magnetic nanoparticles under simulated visible light and solar light. J. Environ. Chem. Eng. 2018, 6, 59–67. [Google Scholar] [CrossRef]
- Zhu, S.; Wang, D. Photocatalysis: Basic Principles, Diverse Forms of Implementations and Emerging Scientific Opportunities. Adv. Energy Mater. 2017, 7, 1–24. [Google Scholar] [CrossRef] [Green Version]
- Akerdi, A.G.; Bahrami, S.H. Application of heterogeneous nano-semiconductors for photocatalytic advanced oxidation of organic compounds: A review. J. Environ. Chem. Eng. 2019, 7, 103283. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nat. Cell Biol. 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Fujishima, A.; Rao, T.N.; Tryk, D.A. Titanium dioxide photocatalysis. J. Photochem. Photobiol. C Photochem. Rev. 2000, 1, 1–21. [Google Scholar] [CrossRef]
- Martha, S.; Sahoo, P.C.; Parida, K.M. An overview on visible light responsive metal oxide based photocatalysts for hydrogen energy production. RSC Adv. 2015, 5, 61535–61553. [Google Scholar] [CrossRef]
- Belachew, N.; Kahsay, M.H.; Tadesse, A.; Basavaiah, K. Green synthesis of reduced graphene oxide grafted Ag/ZnO for photocatalytic abatement of methylene blue and antibacterial activities. J. Environ. Chem. Eng. 2020, 8, 104106. [Google Scholar] [CrossRef]
- Hu, Z.; He, Q.; Ge, M. Photocatalytic degradation of organic contaminants by magnetic Ag3PO4/MFe2O4 (M = Zn, Ni, Co) composites: A comparative study and a new insight into mechanism. J. Mater. Sci. Mater. Electron. 2020, 4. [Google Scholar] [CrossRef]
- Nuengmatcha, P.; Porrawatkul, P.; Chanthai, S.; Sricharoen, P.; Limchoowong, N.; Sricharoen, P. Enhanced photocatalytic degradation of methylene blue using Fe2O3/graphene/CuO nanocomposites under visible light. J. Environ. Chem. Eng. 2019, 7, 103438. [Google Scholar] [CrossRef]
- Xu, B.; Ahmed, M.B.; Zhou, J.L.; Altaee, A.; Wu, M.; Xu, G. Photocatalytic removal of perfluoroalkyl substances from water and wastewater: Mechanism, kinetics and controlling factors. Chemosphere 2017, 189, 717–729. [Google Scholar] [CrossRef] [PubMed]
- Dariani, R.; Esmaeili, A.; Mortezaali, A.; Dehghanpour, S. Photocatalytic reaction and degradation of methylene blue on TiO2 nano-sized particles. Optik 2016, 127, 7143–7154. [Google Scholar] [CrossRef]
- Liu, M.; Yin, W.; Qian, F.-J.; Zhao, T.-L.; Yao, Q.-Z.; Fu, S.-Q.; Zhou, G.-T. A novel synthesis of porous TiO2 nanotubes and sequential application to dye contaminant removal and Cr(VI) visible light catalytic reduction. J. Environ. Chem. Eng. 2020, 8, 104061. [Google Scholar] [CrossRef]
- Xu, C.; Rangaiah, G.P.; Zhao, X.S. Photocatalytic Degradation of Methylene Blue by Titanium Dioxide: Experimental and Modeling Study. Ind. Eng. Chem. Res. 2014, 53, 14641–14649. [Google Scholar] [CrossRef]
- Grande, F.; Tucci, P. Titanium Dioxide Nanoparticles: A Risk for Human Health? Mini Rev. Med. Chem. 2016, 16, 762–769. [Google Scholar] [CrossRef] [PubMed]
- Poulopoulos, S.G.; Yerkinova, A.; Ulykbanova, G.; Inglezakis, V.J. Photocatalytic treatment of organic pollutants in a synthetic wastewater using UV light and combinations of TiO2, H2O2 and Fe (III). PLoS ONE 2019, 14, e0216745. [Google Scholar] [CrossRef]
- Khdary, N.H.; Alkhuraiji, W.S.; Sakthivel, T.S.; Khdary, D.N.; Salam, M.A.; Alshihri, S.; Al-Mayman, S.I.; Seal, S. Synthesis of Superior Visible-Light-Driven Nanophotocatalyst Using High Surface Area TiO2 Nanoparticles Decorated with CuxO Particles. Catalysts 2020, 10, 872. [Google Scholar] [CrossRef]
- Luttrell, T.; Halpegamage, S.; Tao, J.; Kramer, A.; Sutter, E.A.; Batzill, M. Why is anatase a better photocatalyst than rutile?—Model studies on epitaxial TiO2 films. Sci. Rep. 2015, 4, 4043. [Google Scholar] [CrossRef] [Green Version]
- Li, R.; Jia, Y.; Bu, N.; Wu, J.; Zhen, Q. Photocatalytic degradation of methyl blue using Fe2O3/TiO2 composite ceramics. J. Alloys Compd. 2015, 643, 88–93. [Google Scholar] [CrossRef]
- Lazar, M.A.; Varghese, S.; Nair, S.S. Photocatalytic Water Treatment by Titanium Dioxide: Recent Updates. Catalysts 2012, 2, 572–601. [Google Scholar] [CrossRef] [Green Version]
- Su, C.; Lin, K.-F.; Lin, Y.-H.; You, B.-H. Preparation and characterization of high-surface-area titanium dioxide by sol-gel process. J. Porous Mater. 2006, 13, 251–258. [Google Scholar] [CrossRef]
- He, R.; Tsuzuki, T. Synthesis of high surface area amorphous tin-zinc oxides by a sol-gel method. In Proceedings of the 2010 International Conference on Nanoscience and Nanotechnology (ICONN 2010), Sydney, Australia, 22–26 February 2010; pp. 154–157. [Google Scholar] [CrossRef]
- Nateq, M.H.; Ceccato, R. Sol-Gel Synthesis of TiO2 Nanocrystalline Particles with Enhanced Surface Area through the Reverse Micelle Approach. Adv. Mater. Sci. Eng. 2019, 2019, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Picco, S.; Villegas, L.; Tonelli, F.; Merlo, M.; Rigau, J.; Diaz, D.; Masuelli, M. Sol-Gel Processes of Functional Powders and Films. 2016. Available online: https://www.intechopen.com/books/chemical-reactions-in-inorganic-chemistry/sol-gel-processes-of-functional-powders-and-films (accessed on 12 February 2021).
- Ola, O.O.; Maroto-Valer, M.M.; Liu, D.; Mackintosh, S.; Lee, C.-W.; Wu, J.C.S. Performance comparison of CO2 conversion in slurry and monolith photoreactors using Pd and Rh-TiO2 catalyst under ultraviolet irradiation. Appl. Catal. B Environ. 2012, 126, 172–179. [Google Scholar] [CrossRef]
- Yang, G.; Li, C. Electrofiltration of silica nanoparticle-containing wastewater using tubular ceramic membranes. Sep. Purif. Technol. 2007, 58, 159–165. [Google Scholar] [CrossRef]
- Kuvayskaya, A.; Lotsi, B.; Mohseni, R.; Vasiliev, A. Mesoporous adsorbents for perfluorinated compounds. Microporous Mesoporous Mater. 2020, 305, 110374. [Google Scholar] [CrossRef]
- Stebel, E.K.; Pike, K.A.; Nguyen, H.; Hartmann, H.A.; Klonowski, M.J.; Lawrence, M.G.; Collins, R.M.; Hefner, C.E.; Edmiston, P.L. Absorption of short-chain to long-chain perfluoroalkyl substances using swellable organically modified silica. Environ. Sci. Water Res. Technol. 2019, 5, 1854–1866. [Google Scholar] [CrossRef]
- Da Silva, R.C.; Kubaski, E.T.; Tenório-Neto, E.T.; Lima-Tenório, M.K.; Tebcherani, S.M. Foam glass using sodium hydroxide as foaming agent: Study on the reaction mechanism in soda-lime glass matrix. J. Non Cryst. Solids 2019, 511, 177–182. [Google Scholar] [CrossRef]
- ASTM C128-15. Standard Test Method for Relative Density (Specific Gravity) and Absorption of Fine Aggregate; ASTM International: West Conshohocken, PA, USA, 2015; Available online: www.astm.org (accessed on 12 February 2021).
- Mills, A. An overview of the methylene blue ISO test for assessing the activities of photocatalytic films. Appl. Catal. B Environ. 2012, 128, 144–149. [Google Scholar] [CrossRef]
- Gurav, J.L.; Jung, I.-K.; Park, H.-H.; Kang, E.S.; Nadargi, D.Y. Silica Aerogel: Synthesis and Applications. J. Nanomater. 2010, 2010, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Şahin, I.; Özbakır, Y.; Inönü, Z.; Ulker, Z.; Erkey, C. Kinetics of Supercritical Drying of Gels. Gels 2018, 4, 3. [Google Scholar] [CrossRef] [Green Version]
- Levy, D.; Zayat, M. The Sol-Gel Handbook: Synthesis, Characterization, and Applications, 1st ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2015. [Google Scholar]
- Danks, A.; Hall, S.R.; Schnepp, Z. The evolution of ‘sol–gel’ chemistry as a technique for materials synthesis. Mater. Horiz. 2016, 3, 91–112. [Google Scholar] [CrossRef] [Green Version]
- Available online: http://www.iso.org/iso/isocatalogue/cataloguetc/cataloguedetail.htm?csnumber=46019 (accessed on 13 February 2021).
- Nibret, G.; Ahmad, S.; Rao, D.G.; Ahmad, I.; Shaikh, M.A.M.U.; Rehman, Z.U. Removal of Methylene Blue Dye from Textile Wastewater Using Water Hyacinth Activated Carbon as Adsorbent: Synthesis, Characterization and Kinetic Studies. SSRN Electron. J. 2019, 1959–1969. [Google Scholar] [CrossRef]
- Hou, C.; Hu, B.; Zhu, J. Photocatalytic Degradation of Methylene Blue over TiO2 Pretreated with Varying Concentrations of NaOH. Catalysts 2018, 8, 575. [Google Scholar] [CrossRef] [Green Version]
- Mills, A.; Hazafy, D.; Parkinson, J.; Tuttle, T.; Hutchings, M.G. Effect of alkali on methylene blue (C.I. Basic Blue 9) and other thiazine dyes. Dyes Pigment. 2011, 88, 149–155. [Google Scholar] [CrossRef]
- Milošević, M.D.; Logar, M.M.; Poharc-Logar, A.V.; Jakšić, N.L. Orientation and Optical Polarized Spectra (380–900 nm) of Methylene Blue Crystals on a Glass Surface. Int. J. Spectrosc. 2013, 2013, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Pennell, K. Transport and Remediation of Per- and Polyfluoroalkyl Substances (PFAS) in the Subsurface. In press.
- Zhang, W.; Liang, Y. Removal of eight perfluoroalkyl acids from aqueous solutions by aeration and duckweed. Sci. Total Environ. 2020, 724, 138357. [Google Scholar] [CrossRef]















| 5 mg/L | 50 mg/L | 500 mg/L | 1000 mg/L | |
|---|---|---|---|---|
| Intrusion Data Summary | ||||
| Total Intrusion Volume (mL/g) | 0.9984 | 0.8768 | 1.2206 | 1.0791 |
| Total Pore Area (m/g) | 33.152 | 34.260 | 34.450 | 38.723 |
| Median Pore Diameter (Volume, nm) | 750.9 | 1162.6 | 1249.6 | 1014.0 |
| Median Pore Diameter (Area, nm2) | 17.4 | 14.9 | 16.3 | 16.0 |
| Average Pore Diameter (4 V/A, nm) | 120.5 | 102.4 | 141.7 | 111.5 |
| Bulk Density at 0.20 psia (g/mL) | 0.5349 | 0.5981 | 0.4767 | 0.4998 |
| Apparent (skeletal) Density (g/mL) | 1.1478 | 1.2576 | 1.1399 | 1.0849 |
| Porosity (%) | 53.4006 | 52.4428 | 58.1829 | 53.9323 |
| Stem Volume Used (%) | 57 | 46 | 61 | 59 |
| Pore Structure Summary | ||||
| BET Surface Area (m2/g) | 162.3000 | 133.9600 | 129.2300 | 156.9500 |
| Tortuosity factor | 0.032 | 0.065 | 0.044 | 0.032 |
| Tortuosity | 0.9166 | 1.3234 | 1.1439 | 1.2185 |
| Mayer Stowe Summary | ||||
| Interstitial porosity (%) | 35.0388 | 36.3433 | 28.8441 | 25.9500 |
| Silicic Concentration of SGM (mg/L) | ln(C0/C) = kt + b | R2 | k (min−1) |
|---|---|---|---|
| 5 | ln(C0/C) = 0.0286t − 0.131 | 0.924 | 0.0286 |
| 50 | ln(C0/C) = 0.0385t − 0.278 | 0.8882 | 0.0385 |
| 500 | ln(C0/C) = 0.045t − 0.2114 | 0.9522 | 0.045 |
| 1000 | ln(C0/C) = 0.0337t − 0.2342 | 0.9054 | 0.0337 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
McIntyre, H.M.; Hart, M.L. Photocatalytic Porous Silica-Based Granular Media for Organic Pollutant Degradation in Industrial Waste-Streams. Catalysts 2021, 11, 258. https://doi.org/10.3390/catal11020258
McIntyre HM, Hart ML. Photocatalytic Porous Silica-Based Granular Media for Organic Pollutant Degradation in Industrial Waste-Streams. Catalysts. 2021; 11(2):258. https://doi.org/10.3390/catal11020258
Chicago/Turabian StyleMcIntyre, Hannah M., and Megan L. Hart. 2021. "Photocatalytic Porous Silica-Based Granular Media for Organic Pollutant Degradation in Industrial Waste-Streams" Catalysts 11, no. 2: 258. https://doi.org/10.3390/catal11020258