Versatile Coordination Polymer Catalyst for Acid Reactions Involving Biobased Heterocyclic Chemicals
Abstract
:1. Introduction
2. Results and Discussion
2.1. General Considerations
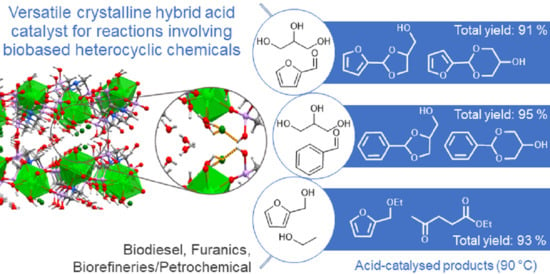
2.2. Reaction of Benzaldehyde and Glycerol to Heterobicyclic Products
2.3. Reaction of Furfural and Glycerol to Heterobicyclic Products
2.4. Types of Active Species and Mechanistic Insights
2.5. Catalyst Stability and Structural Characterization
2.6. Other Biobased Systems Involving Heterocyclic Compounds
3. Materials and Methods
3.1. Catalyst Synthesis and Characterization
3.2. Catalytic Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Grand New Research, Glycerol Market Size, Share and Trends Analysis Report by Source (Biodiesel, Fatty Acids, Fatty Alcohols, Soap), by Type (Crude, Refined) by End Use (Food and Beverage, Pharmaceutical), by Region, and Segment Forecasts, 2020–2027. 2020. Available online: https://www.grandviewresearch.com/industry-analysis/glycerol-market (accessed on 8 January 2021).
- ReportLinker, Glycerol Market Size, Share and Trends Analysis Report by Source, by Type, by End Use, by Region and Segment Forecasts, 2020–2027. 2020. Available online: https://www.reportlinker.com/p05930634/.html?utm_source=GNW (accessed on 8 January 2021).
- Smirnov, A.A.; Selishcheva, S.A.; Yakovlev, V.A. Acetalization Catalysts for Synthesis of Valuable Oxygenated Fuel Additives from Glycerol. Catalysts 2018, 8, 595. [Google Scholar] [CrossRef] [Green Version]
- Natalia, B.; Thomas, R.; Martin, K.; Stephan, A.S. Valorisation of Glycerol as Renewable Feedstock: Comparison of the Exploration of Chemical Transformation Methods Aided by High Throughput Experimentation. Comb. Chem. High. Throughput Screen. 2012, 15, 123–135. [Google Scholar] [CrossRef]
- Rodrigues, A.; Bordado, J.C.; dos Santos, R.G. Upgrading the Glycerol from Biodiesel Production as a Source of Energy Carriers and Chemicals—A Technological Review for Three Chemical Pathways. Energies 2017, 10, 1817. [Google Scholar] [CrossRef] [Green Version]
- Rahmat, N.; Abdullah, A.Z.; Mohamed, A.R. Recent progress on innovative and potential technologies for glycerol transformation into fuel additives: A critical review. Renew. Sustain. Energy Rev. 2010, 14, 987–1000. [Google Scholar] [CrossRef]
- Checa, M.; Nogales-Delgado, S.; Montes, V.; Encinar, J.M. Recent Advances in Glycerol Catalytic Valorization: A Review. Catalysts 2020, 10, 1279. [Google Scholar] [CrossRef]
- Kosamia, N.M.; Samavi, M.; Uprety, B.K.; Rakshit, S.K. Valorization of Biodiesel Byproduct Crude Glycerol for the Production of Bioenergy and Biochemicals. Catalysts 2020, 10, 609. [Google Scholar] [CrossRef]
- Zhou, C.-H.; Beltramini, J.N.; Lin, C.-X.; Xu, Z.-P.; Lu, G.Q.; Tanksale, A. Selective oxidation of biorenewable glycerol with molecular oxygen over Cu-containing layered double hydroxide-based catalysts. Catal. Sci. Technol. 2011, 1, 111–122. [Google Scholar] [CrossRef]
- Walgode, P.M.; Faria, R.P.V.; Rodrigues, A.E. A review of aerobic glycerol oxidation processes using heterogeneous catalysts: A sustainable pathway for the production of dihydroxyacetone. Catal. Rev. 2020. [Google Scholar] [CrossRef]
- Beltrán Prieto, J.C.; Kolomazník, K.; Pecha, J. A Review of Catalytic Systems for Glycerol Oxidation: Alternatives for Waste Valorization. Aust. J. Chem. 2013, 66, 511–521. [Google Scholar] [CrossRef]
- Lari, G.M.; Mondelli, C.; Pérez-Ramírez, J. Gas-phase oxidation of glycerol to dihydroxyacetone over tailored iron zeolites. ACS Catal. 2015, 5, 1453–1461. [Google Scholar] [CrossRef]
- Palacio, R.; Amaya, Á.A.; Blach, D.; Torres, S.; Hernández, D.; López, D.; Martinez, F. Influence of the Acid Properties of the Support on Au-Based Catalysts for Glycerol Oxidation in Aqueous Medium. ChemistrySelect 2020, 5, 7789–7796. [Google Scholar] [CrossRef]
- Liu, L.; Ye, X.P.; Katryniok, B.; Capron, M.; Paul, S.; Dumeignil, F. Extending Catalyst Life in Glycerol-to-Acrolein Conversion Using Non-thermal Plasma. Front. Chem. 2019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dodekatos, G.; Abis, L.; Freakley, S.J.; Tüysüz, H.; Hutchings, G.J. Glycerol Oxidation Using MgO-and Al2O3-supported Gold and Gold–Palladium Nanoparticles Prepared in the Absence of Polymer Stabilizers. ChemCatChem 2018, 10, 1351–1359. [Google Scholar] [CrossRef]
- Farnetti, E.; Crotti, C. Selective oxidation of glycerol to formic acid catalyzed by iron salts. Catal. Commun. 2016, 84, 1–4. [Google Scholar] [CrossRef]
- Crotti, C.; Farnetti, E.J. Selective oxidation of glycerol catalyzed by iron complexes. Mol. Catal. A Chem. 2015, 396, 353–359. [Google Scholar] [CrossRef]
- Xu, C.; Gan, J.; Mei, X.; Zhou, Y.; Duanmu, J.; Zhu, G.; Zhang, H.; Han, X.; Wang, Y.; Liu, S.-B. Highly Active Silver ion-Exchanged Silicotungstic Acid Catalysts for Selective Esterification of Glycerol with Lauric Acid. Catal. Lett. 2020, 150, 3584–3597. [Google Scholar] [CrossRef]
- Narkhede, N.; Patel, A. Sustainable valorisation of glycerol via acetalization as well as carboxylation reactions over silicotungstates anchored to zeolite Hβ. Appl. Catal. A Gen. 2016, 515, 154–163. [Google Scholar] [CrossRef]
- Florez-Rodriguez, P.P.; Pamphile-Adrián, A.J.; Passos, F.B. Glycerol conversion in the presence of carbon dioxide on alumina supported nickel catalyst. Catal. Today 2014, 237, 38–46. [Google Scholar] [CrossRef]
- Barrault, J.; Jerome, F. Design of new solid catalysts for the selective conversion of glycerol. Eur. J. Lipid Sci. Technol. 2008, 110, 825–830. [Google Scholar] [CrossRef]
- Oprescu, E.E.; Stepan, E.; Rosca, P.; Radu, A.; Enascuta, C. Synthesis of glycerol carbonate over hydrotalcite catalyst. Rev. De Chim. 2012, 63, 621–625. [Google Scholar]
- Ruppert, A.M.; Meeldijk, J.D.; Kuipers, B.W.; Erné, B.H.; Weckhuysen, B.M. Glycerol etherification over highly active CaO-based materials: New mechanistic aspects and related colloidal particle formation. Chem. (Weinh. Der Bergstr. Ger.) 2008, 14, 2016–2024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aguado-Deblas, L.; Estevez, R.; Russo, M.; La Parola, V.; Bautista, F.M.; Testa, M.L. Microwave-Assisted Glycerol Etherification Over Sulfonic Acid Catalysts. Materials 2020, 13, 1584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guerrero-Urbaneja, P.; García-Sancho, C.; Moreno-Tost, R.; Mérida-Robles, J.; Santamaría-González, J.; Jiménez-López, A.; Maireles-Torres, P. Glycerol valorization by etherification to polyglycerols by using metal oxides derived from MgFe hydrotalcites. Appl. Catal. A Gen. 2014, 470, 199–207. [Google Scholar] [CrossRef]
- Clacens, J.M.; Pouilloux, Y.; Barrault, J. Selective etherification of glycerol to polyglycerols over impregnated basic MCM-41 type mesoporous catalysts. Appl. Catal. A Gen. 2002, 227, 181–190. [Google Scholar] [CrossRef]
- Magar, S.; Mohanraj, G.T.; Jana, S.K.; Rode, C.V. Synthesis and characterization of supported heteropoly acid: Efficient solid acid catalyst for glycerol esterification to produce biofuel additives. Inorg. Nano-Met. Chem. 2020, 50, 1157–1165. [Google Scholar] [CrossRef]
- Reinoso, D.M.; Boldrini, D.E. Kinetic study of fuel bio-additive synthesis from glycerol esterification with acetic acid over acid polymeric resin as catalyst. Fuel 2020, 264, 116879. [Google Scholar] [CrossRef]
- Aghbashlo, M.; Tabatabaei, M.; Rastegari, H.; Ghaziaskar, H.S.; Valijanian, E. Exergy-based optimization of a continuous reactor applied to produce value-added chemicals from glycerol through esterification with acetic acid. Energy 2018, 150, 351–362. [Google Scholar] [CrossRef]
- Popova, M.; Lazarova, H.; Kalvachev, Y.; Todorova, T.; Szegedi, Á.; Shestakova, P.; Mali, G.; Dasireddy, V.D.B.C.; Likozar, B. Zr-modified hierarchical mordenite as heterogeneous catalyst for glycerol esterification. Catal. Commun. 2017, 100, 10–14. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez-Arellano, C.; Arancon, R.A.D.; Luque, R. Al-SBA-15 catalysed cross-esterification and acetalisation of biomass-derived platform chemicals. Green Chem. 2014, 16, 4985–4993. [Google Scholar] [CrossRef]
- Gonzalez-Arellano, C.; De, S.; Luque, R. Selective glycerol transformations to high value-added products catalysed by aluminosilicate-supported iron oxide nanoparticles. Catal. Sci. Technol. 2014, 4, 4242–4249. [Google Scholar] [CrossRef]
- Mallesham, B.; Sudarsanam, P.; Reddy, B.M. Production of Biofuel Additives from Esterification and Acetalization of Bioglycerol over SnO2-Based Solid Acids. Ind. Eng. Chem. Res. 2014, 53, 18775–18785. [Google Scholar] [CrossRef]
- Rastegari, H.; Ghaziaskar, H.S.; Yalpani, M. Valorization of Biodiesel Derived Glycerol to Acetins by Continuous Esterification in Acetic Acid: Focusing on High Selectivity to Diacetin and Triacetin with No Byproducts. Ind. Eng. Chem. Res. 2015, 54, 3279–3284. [Google Scholar] [CrossRef]
- Mallesham, B.; Govinda Rao, B.; Reddy, B.M. Production of biofuel additives by esterification and acetalization of bioglycerol. Comptes Rendus Chim. 2016, 19, 1194–1202. [Google Scholar] [CrossRef]
- Zhu, S.; Gao, X.; Dong, F.; Zhu, Y.; Zheng, H.; Li, Y. Design of a highly active silver-exchanged phosphotungstic acid catalyst for glycerol esterification with acetic acid. J. Catal. 2013, 306, 155–163. [Google Scholar] [CrossRef]
- Gonzalez-Arellano, C.; Parra-Rodriguez, L.; Luque, R. Mesoporous Zr–SBA-16 catalysts for glycerol valorization processes: Towards biorenewable formulations. Catal. Sci. Technol. 2014, 4, 2287–2292. [Google Scholar] [CrossRef]
- Okoye, P.U.; Wang, S.; Khanday, W.A.; Li, S.; Tang, T.; Zhang, L. Box-Behnken optimization of glycerol transesterification reaction to glycerol carbonate over calcined oil palm fuel ash derived catalyst. Renew. Energy 2020, 146, 2676–2687. [Google Scholar] [CrossRef]
- Devarajan, A.; Thiripuranthagan, S.; Radhakrishnan, R.; Kumaravel, S. Solvent Free Transesterification of Glycerol Into Glycerol Carbonate Over Nanostructured CaAl Hydrotalcite Catalyst. J. Nanosci. Nanotechnol. 2018, 18, 4588–4599. [Google Scholar] [CrossRef]
- Shafiei, A.; Rastegari, H.; Ghaziaskar, H.S.; Yalpani, M. Glycerol transesterification with ethyl acetate to synthesize acetins using ethyl acetate as reactant and entrainer. Biofuel Res. J. 2017, 4, 565–570. [Google Scholar] [CrossRef] [Green Version]
- Morales, G.; Paniagua, M.; Melero, J.A.; Vicente, G.; Ochoa, C. Sulfonic Acid-Functionalized Catalysts for the Valorization of Glycerol via Transesterification with Methyl Acetate. Ind. Eng. Chem. Res. 2011, 50, 5898–5906. [Google Scholar] [CrossRef]
- Simón, D.; Borreguero, A.M.; de Lucas, A.; Rodríguez, J.F. Valorization of crude glycerol as a novel transesterification agent in the glycolysis of polyurethane foam waste. Polym. Degrad. Stab. 2015, 121, 126–136. [Google Scholar] [CrossRef]
- Esteban, J.; Domínguez, E.; Ladero, M.; Garcia-Ochoa, F. Kinetics of the production of glycerol carbonate by transesterification of glycerol with dimethyl and ethylene carbonate using potassium methoxide, a highly active catalyst. Fuel Process. Technol. 2015, 138, 243–251. [Google Scholar] [CrossRef]
- Tavor, D.; Sheviev, O.; Dlugy, C.; Wolfson, A. Transfer hydrogenations of benzaldehyde using glycerol as solvent and hydrogen source. Can. J. Chem. 2010, 88, 305–308. [Google Scholar] [CrossRef]
- Crabtree, R.H. Transfer Hydrogenation with Glycerol as H-Donor: Catalyst Activation, Deactivation and Homogeneity. Acs Sustain. Chem. Eng. 2019, 7, 15845–15853. [Google Scholar] [CrossRef]
- Crotti, C.; Kašpar, J.; Farnetti, E. Dehydrogenation of glycerol to dihydroxyacetone catalyzed by iridium complexes with P–N ligands. Green Chem. 2010, 12, 1295–1300. [Google Scholar] [CrossRef]
- Farnetti, E.; Kašpar, J.; Crotti, C. A novel glycerol valorization route: Chemoselective dehydrogenation catalyzed by iridium derivatives. Green Chem. 2009, 11, 704–709. [Google Scholar] [CrossRef]
- Dusescu, C.; Bolocan, I. New catalysts for the glycerol hydrogenolysis. Rev. De Chim. 2012, 63, 732–738. [Google Scholar]
- Liang, Y.; Shi, G.; Jin, K. Promotion Effect of Al2O3 on Pt–WOx/SiO2 Catalysts for Selective Hydrogenolysis of Bioglycerol to 1,3-Propanediol in Liquid Phase. Catal. Lett. 2020, 150, 2365–2376. [Google Scholar] [CrossRef]
- Lei, N.; Zhao, X.; Hou, B.; Yang, M.; Zhou, M.; Liu, F.; Wang, A.; Zhang, T. Effective Hydrogenolysis of Glycerol to 1,3-Propanediol over Metal-Acid Concerted Pt/WOx/Al2O3 Catalysts. ChemCatChem 2019, 11, 3903–3912. [Google Scholar] [CrossRef]
- Von Held Soares, A.; Atia, H.; Armbruster, U.; Passos, F.B.; Martin, A. Platinum, palladium and nickel supported on Fe3O4 as catalysts for glycerol aqueous-phase hydrogenolysis and reforming. Appl. Catal. A Gen. 2017, 548, 179–190. [Google Scholar] [CrossRef]
- Mallesham, B.; Sudarsanam, P.; Reddy, B.V.S.; Reddy, B.M. Development of cerium promoted copper–magnesium catalysts for biomass valorization: Selective hydrogenolysis of bioglycerol. Appl. Catal. B Environ. 2016, 181, 47–57. [Google Scholar] [CrossRef]
- Mauriello, F.; Ariga, H.; Musolino, M.G.; Pietropaolo, R.; Takakusagi, S.; Asakura, K. Exploring the catalytic properties of supported palladium catalysts in the transfer hydrogenolysis of glycerol. Appl. Catal. B Environ. 2015, 166, 121–131. [Google Scholar] [CrossRef]
- Lari, G.M.; García-Muelas, R.; Mondelli, C.; López, N.; Pérez-Ramírez, J. Glycerol oxidehydration to pyruvaldehyde over silver-based catalysts for improved lactic acid production. Green Chem. 2016, 18, 4682–4692. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Wang, B.; Du, Y.; Borgna, A. Supported H4SiW12O40/Al2O3 solid acid catalysts for dehydration of glycerol to acrolein: Evolution of catalyst structure and performance with calcination temperature. Appl. Catal. A Gen. 2015, 489, 32–41. [Google Scholar] [CrossRef]
- Rao, G.S.; Rajan, N.P.; Pavankumar, V.; Chary, K.V.R. Vapour phase dehydration of glycerol to acrolein over NbOPO4 catalysts. J. Chem. Technol. Biotechnol. 2014, 89, 1890–1897. [Google Scholar] [CrossRef]
- Yadav, G.D.; Sharma, R.V.; Katole, S.O. Selective Dehydration of Glycerol to Acrolein: Development of Efficient and Robust Solid Acid Catalyst MUICaT-5. Ind. Eng. Chem. Res. 2013, 52, 10133–10144. [Google Scholar] [CrossRef]
- Shiju, N.R.; Brown, D.R.; Wilson, K.; Rothenberg, G. Glycerol Valorization: Dehydration to Acrolein Over Silica-Supported Niobia Catalysts. Top. Catal. 2010, 53, 1217–1223. [Google Scholar] [CrossRef] [Green Version]
- Katryniok, B.; Paul, S.; Capron, M.; Dumeignil, F. Towards the sustainable production of acrolein by glycerol dehydration. ChemSusChem 2009, 2, 719–730. [Google Scholar] [CrossRef]
- Caputo, D.; Casiello, M.; Milella, A.; Oberhauser, W.; Maffezzoli, A.; Nacci, A.; Fusco, C.; D’Accolti, L. Deep Control of Linear Oligomerization of Glycerol Using Lanthanum Catalyst on Mesoporous Silica Gel. Catalysts 2020, 10, 1170. [Google Scholar] [CrossRef]
- Zanoni, A.; Gardoni, G.; Sponchioni, M.; Moscatelli, D. Valorisation of glycerol and CO2 to produce biodegradable polymer nanoparticles with a high percentage of bio-based components. J. CO2 Util. 2020, 40, 101192. [Google Scholar] [CrossRef]
- Barros, F.J.S.; Cecilia, J.A.; Moreno-Tost, R.; de Oliveira, M.F.; Rodríguez-Castellón, E.; Luna, F.M.T.; Vieira, R.S. Glycerol Oligomerization Using Low Cost Dolomite Catalyst. Waste Biomass Valorization 2020, 11, 1499–1512. [Google Scholar] [CrossRef]
- Pham, P.D.; Monge, S.; Lapinte, V.; Raoul, Y.; Robin, J.J. Glycerol-based co-oligomers by free-radical chain transfer polymerization: Towards reactive polymers bearing acetal and/or carbonate groups with enhanced properties. Eur. Polym. J. 2017, 95, 491–502. [Google Scholar] [CrossRef] [Green Version]
- Barrault, J.; Clacens, J.M.; Pouilloux, Y. Selective Oligomerization of Glycerol Over Mesoporous Catalysts. Top. Catal. 2004, 27, 137–142. [Google Scholar] [CrossRef]
- Zhang, H.; Li, H.; Wang, A.; Xu, C.; Yang, S. Progress of Catalytic Valorization of Bio-Glycerol with Urea into Glycerol Carbonate as a Monomer for Polymeric Materials. Adv. Polym. Technol. 2020, 2020, 7207068. [Google Scholar] [CrossRef] [Green Version]
- Jang, H.-S.; Bae, K.; Shin, M.; Kim, S.M.; Kim, C.-U.; Suh, Y.-W. Aromatization of glycerol/alcohol mixtures over zeolite H-ZSM-5. Fuel 2014, 134, 439–447. [Google Scholar] [CrossRef]
- Torres, D.; Arcelus-Arrillaga, P.; Millan, M.; Pinilla, J.L.; Suelves, I. Enhanced Reduction of Few-Layer Graphene Oxide via Supercritical Water Gasification of Glycerol. Nanomaterials 2017, 7, 447. [Google Scholar] [CrossRef] [Green Version]
- Almeida, A.; Ribeiro, A.; Ramalho, E.; Pilão, R. Crude glycerol gasification in a fixed bed gasifier. Energy Procedia 2018, 153, 149–153. [Google Scholar] [CrossRef]
- Charisiou, N.D.; Siakavelas, G.I.; Papageridis, K.N.; Motta, D.; Dimitratos, N.; Sebastian, V.; Polychronopoulou, K.; Goula, M.A. The Effect of Noble Metal (M: Ir, Pt, Pd) on M/Ce2O3-γ-Al2O3 Catalysts for Hydrogen Production via the Steam Reforming of Glycerol. Catalysts 2020, 10, 790. [Google Scholar] [CrossRef]
- Bac, S.; Keskin, S.; Avci, A.K. Recent advances in sustainable syngas production by catalytic CO2 reforming of ethanol and glycerol. Sustain. Energy Fuels 2020, 4, 1029–1047. [Google Scholar] [CrossRef]
- Remón, J.; Jarauta-Córdoba, C.; García, L.; Arauzo, J. Effect of acid (CH3COOH, H2SO4 and H3PO4) and basic (KOH and NaOH) impurities on glycerol valorisation by aqueous phase reforming. Appl. Catal. B Environ. 2017, 219, 362–371. [Google Scholar] [CrossRef]
- Esteve-Adell, I.; Crapart, B.; Primo, A.; Serp, P.; Garcia, H. Aqueous phase reforming of glycerol using doped graphenes as metal-free catalysts. Green Chem. 2017, 19, 3061–3068. [Google Scholar] [CrossRef]
- Remón, J.; Giménez, J.R.; Valiente, A.; García, L.; Arauzo, J. Production of gaseous and liquid chemicals by aqueous phase reforming of crude glycerol: Influence of operating conditions on the process. Energy Convers. Manag. 2016, 110, 90–112. [Google Scholar] [CrossRef] [Green Version]
- Iliuta, I.; Iliuta, M.C. Integration of sorption-enhanced steam glycerol reforming with methanation of in-situ removed carbon dioxide-An alternative for glycerol valorization. Int. J. Hydrog. Energy 2020, 45, 18574–18586. [Google Scholar] [CrossRef]
- Roslan, N.A.; Abidin, S.Z.; Ideris, A.; Vo, D.-V.N. A review on glycerol reforming processes over Ni-based catalyst for hydrogen and syngas productions. Int. J. Hydrog. Energy 2020, 45, 18466–18489. [Google Scholar] [CrossRef]
- Lin, Y.-C. Catalytic valorization of glycerol to hydrogen and syngas. Int. J. Hydrog. Energy 2013, 38, 2678–2700. [Google Scholar] [CrossRef]
- Dodson, J.R.; Avellar, T.; Athayde, J.; Mota, C.J.A. Glycerol acetals with antioxidant properties. Pure Appl. Chem. 2014, 86, 905–911. [Google Scholar] [CrossRef]
- Mallesham, B.; Sudarsanam, P.; Reddy, B.M. Eco-friendly synthesis of bio-additive fuels from renewable glycerol using nanocrystalline SnO2-based solid acids. Catal. Sci. Technol. 2014, 4, 803–813. [Google Scholar] [CrossRef]
- Deutsch, J.; Martin, A.; Lieske, H. Investigations on heterogeneously catalysed condensations of glycerol to cyclic acetals. J. Catal. 2007, 245, 428–435. [Google Scholar] [CrossRef]
- Leng, Y.; Zhao, J.; Jiang, P.; Lu, D. POSS-derived solid acid catalysts with excellent hydrophobicity for highly efficient transformations of glycerol. Catal. Sci. Technol. 2016, 6, 875–881. [Google Scholar] [CrossRef]
- Mallesham, B.; Sudarsanam, P.; Raju, G.; Reddy, B.M. Design of highly efficient Mo and W-promoted SnO2 solid acids for heterogeneous catalysis: Acetalization of bio-glycerol. Green Chem. 2013, 15, 478–489. [Google Scholar] [CrossRef]
- Manjunathan, P.; Marakatti, V.S.; Chandra, P.; Kulal, A.B.; Umbarkar, S.B.; Ravishankar, R.; Shanbhag, G.V. Mesoporous tin oxide: An efficient catalyst with versatile applications in acid and oxidation catalysis. Catal. Today 2018, 309, 61–76. [Google Scholar] [CrossRef]
- Udayakumar, V.; Pandurangan, A. Synthesis of Hf/SBA-15 Lewis acid catalyst for converting glycerol to value-added chemicals. J. Porous Mater. 2017, 24, 979–990. [Google Scholar] [CrossRef]
- Kundu, S.K.; Singuru, R.; Hayashi, T.; Hijikata, Y.; Irle, S.; Mondal, J. Constructing Sulfonic Acid Functionalized Anthracene Derived Conjugated Porous Organic Polymer for Efficient Metal-Free Catalytic Acetalization of Bio-Glycerol. ChemistrySelect 2017, 2, 4705–4716. [Google Scholar] [CrossRef]
- Poly, S.S.; Jamil, M.A.R.; Touchy, A.S.; Yasumura, S.; Siddiki, S.M.A.H.; Toyao, T.; Maeno, Z.; Shimizu, K.-I. Acetalization of glycerol with ketones and aldehydes catalyzed by high silica Hβ zeolite. Mol. Catal. 2019, 479, 110608. [Google Scholar] [CrossRef]
- Pawar, R.R.; Gosai, K.A.; Bhatt, A.S.; Kumaresan, S.; Lee, S.M.; Bajaj, H.C. Clay catalysed rapid valorization of glycerol towards cyclic acetals and ketals. RSC Adv. 2015, 5, 83985–83996. [Google Scholar] [CrossRef]
- Elena-Emilia, O.; Bombos, D.; Bolocan, I.; Dragomir, R.; Rosca, P. Diesel Fuel Green Additive based on Glycerol. Rev. De Chim. Buchar. Orig. Ed. 2014, 65, 1226–1229. [Google Scholar]
- Narkhede, N.; Patel, A. Room temperature acetalization of glycerol to cyclic acetals over anchored silicotungstates under solvent free conditions. RSC Adv. 2014, 4, 19294–19301. [Google Scholar] [CrossRef]
- Faria, R.P.V.; Pereira, C.S.M.; Silva, V.M.T.M.; Loureiro, J.M.; Rodrigues, A.E. Glycerol Valorization as Biofuel: Thermodynamic and Kinetic Study of the Acetalization of Glycerol with Acetaldehyde. Ind. Eng. Chem. Res. 2013, 52, 1538–1547. [Google Scholar] [CrossRef]
- Crotti, C.; Farnetti, E.; Guidolin, N. Alternative intermediates for glycerol valorization: Iridium-catalyzed formation of acetals and ketals. Green Chem. 2010, 12, 2225–2231. [Google Scholar] [CrossRef]
- Ruiz, V.R.; Velty, A.; Santos, L.L.; Leyva-Pérez, A.; Sabater, M.J.; Iborra, S.; Corma, A. Gold catalysts and solid catalysts for biomass transformations: Valorization of glycerol and glycerol–water mixtures through formation of cyclic acetals. J. Catal. 2010, 271, 351–357. [Google Scholar] [CrossRef]
- Han, X.; Yan, W.; Chen, K.; Hung, C.-T.; Liu, L.-L.; Wu, P.-H.; Huang, S.-J.; Liu, S.-B. Heteropolyacid-based ionic liquids as effective catalysts for the synthesis of benzaldehyde glycol acetal. Appl. Catal. A Gen. 2014, 485, 149–156. [Google Scholar]
- Umbarkar, S.B.; Kotbagi, T.V.; Biradar, A.V.; Pasricha, R.; Chanale, J.; Dongare, M.K.; Mamede, A.-S.; Lancelot, C.; Payen, E.J. Acetalization of glycerol using mesoporous MoO3/SiO2 solid acid catalyst. Mol. Catal. A Chem. 2009, 310, 150–158. [Google Scholar] [CrossRef]
- Talebian-Kiakalaieh, A.; Amin, N.A.S.; Najaafi, N.; Tarighi, S. A Review on the Catalytic Acetalization of Bio-renewable Glycerol to Fuel Additives. Front. Chem. 2018, 6, 573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yip, L.; Kubczyk, T.M.; Davies, T.E.; Taylor, S.H.; Apperley, D.C.; Graham, A.E. Nanoporous aluminosilicate mediated transacetalization reactions: Application in glycerol valorization. Catal. Sci.Technol. 2012, 2, 2258–2263. [Google Scholar] [CrossRef]
- Nanda, M.R.; Zhang, Y.; Yuan, Z.; Qin, W.; Ghaziaskar, H.S.; Xu, C. Catalytic conversion of glycerol for sustainable production of solketal as a fuel additive: A review. Renew. Sust. Energ. Rev. 2016, 56, 1022–1031. [Google Scholar] [CrossRef]
- Moity, L.; Benazzouz, A.; Molinier, V.; Nardello-Rataj, V.; Elmkaddem, M.K.; de Caro, P.; Thiébaud-Roux, S.; Gerbaud, V.; Marion, P.; Aubry, J.-M. Glycerol acetals and ketals as bio-based solvents: Positioning in Hansen and COSMO-RS spaces, volatility and stability towards hydrolysis and autoxidation. Green Chem. 2015, 17, 1779–1792. [Google Scholar] [CrossRef] [Green Version]
- Stepan, E.; Enascuta, C.-E.; Oprescu, E.-E.; Radu, E.; Vasilievici, G.; Radu, A.; Stoica, R.; Velea, S.; Nicolescu, A.; Lavric, V. A versatile method for obtaining new oxygenated fuel components from biomass. Ind. Crop. Prod. 2018, 113, 288–297. [Google Scholar] [CrossRef]
- Wegenhart, B.L.; Abu-Omar, M.M. A Solvent-Free Method for Making Dioxolane and Dioxane from the Biorenewables Glycerol and Furfural Catalyzed by Oxorhenium(V) Oxazoline. Inorg. Chem. 2010, 49, 4741–4743. [Google Scholar] [CrossRef]
- Climent, M.J.; Corma, A.; Velty, A. Synthesis of hyacinth, vanilla, and blossom orange fragrances: The benefit of using zeolites and delaminated zeolites as catalysts. Appl. Catal. A Gen. 2004, 263, 155–161. [Google Scholar] [CrossRef]
- Gutiérrez-Acebo, E.; Guerrero-Ruiz, F.; Centenero, M.; Martínez, J.S.; Salagre, P.; Cesteros, Y. Effect of using microwaves for catalysts preparation on the catalytic acetalization of glycerol with furfural to obtain fuel additives. Open Chem. 2018, 16, 386–392. [Google Scholar]
- Adam, F.; Hassan, H.E.; Hello, K.M. The synthesis of N-heterocyclic carbene–silica nano-particles and its catalytic activity in the cyclization of glycerol. J. Taiwan Inst. Chem. E. 2012, 43, 619–630. [Google Scholar] [CrossRef]
- Konwar, L.J.; Samikannu, A.; Mäki-Arvela, P.; Boström, D.; Mikkola, J.-P. Lignosulfonate-based macro/mesoporous solid protonic acids for acetalization of glycerol to bio-additives. Appl. Catal. B Environ. 2018, 220, 314–323. [Google Scholar] [CrossRef]
- Zeitsch, K.J. The Chemistry and Technology of Furfural and Its Many by-Products, 13th ed; Sugar Series; Elsevier: Amesterdam, The Netherlands, 2000. [Google Scholar]
- Vasiliou, A.K.; Kim, J.H.; Ormond, T.K.; Piech, K.M.; Urness, K.N.; Scheer, A.M.; Robichaud, D.J.; Mukarakate, C.; Nimlos, M.R.; Daily, J.W.; et al. Biomass pyrolysis: Thermal decomposition mechanisms of furfural and benzaldehyde. J. Chem. Phys. 2013, 139, 104310. [Google Scholar] [CrossRef] [PubMed]
- Patil, A.; Shinde, S.; Kamble, S.; Rode, C.V. Two-Step Sequence of Acetalization and Hydrogenation for Synthesis of Diesel Fuel Additives from Furfural and Diols. Energy Fuels 2019, 33, 7466–7472. [Google Scholar] [CrossRef]
- Pawar, R.R.; Jadhav, S.V.; Bajaj, H.C. Microwave-assisted rapid valorization of glycerol towards acetals and ketals. Chem. Eng. J. 2014, 235, 61–66. [Google Scholar]
- Ferreira, G.K.B.; Carvalho, C.; Nakagaki, S. Studies of the Catalytic Activity of Iron (III) Porphyrins for the Protection of Carbonyl Groups in Homogeneous Media. Catalysts 2019, 9, 334. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; Shen, Y.; Sun, J.; Xu, F.; Sun, R. Conversion of platform chemical glycerol to cyclic acetals promoted by acidic ionic liquids. RSC Adv. 2014, 4, 18917–18923. [Google Scholar] [CrossRef]
- Yamamoto, K.; Kiyan, A.M.; Bagio, J.C.; Rossi, K.A.B.; Berezuk, F.D.; Berezuk, M.E. Green cyclic acetals production by glycerol etherification reaction with benzaldehyde using cationic acidic resin. Green Process. Synth. 2019, 8, 183–190. [Google Scholar] [CrossRef]
- Sudarsanam, P.; Mallesham, B.; Prasad, A.N.; Reddy, P.S.; Reddy, B.M. Synthesis of bio–additive fuels from acetalization of glycerol with benzaldehyde over molybdenum promoted green solid acid catalysts. Fuel Process. Technol. 2013, 106, 539–545. [Google Scholar] [CrossRef]
- Adam, F.; Batagarawa, M.S.; Hello, K.M.; Al-Juaid, S.S. One-step synthesis of solid sulfonic acid catalyst and its application in the acetalization of glycerol: Crystal structure of cis-5-hydroxy-2-phenyl-1,3-dioxane trimer. Chem. Pap. 2012, 66, 1048–1058. [Google Scholar]
- Patel, A.; Pithadia, D. Low temperature synthesis of bio-fuel additives via valorisation of glycerol with benzaldehyde as well as furfural over a novel sustainable catalyst, 12-tungstosilicic acid anchored to ordered cubic nano-porous MCM-48. Appl. Catal. A Gen. 2020, 602, 117729. [Google Scholar] [CrossRef]
- Wegenhart, B.L.; Liu, S.; Thom, M.; Stanley, D.; Abu-Omar, M.M. Solvent-Free Methods for Making Acetals Derived from Glycerol and Furfural and Their Use as a Biodiesel Fuel Component. ACS Catal. 2012, 2, 2524–2530. [Google Scholar] [CrossRef]
- Bombos, D.; Velea, S.; Bombos, M.; Vasilievici, G.; Oprescu, E. Ecological Component for Motor Fuels Based on Furfural Derivates. Rev. Chim-Bucharest 2016, 67, 745–750. [Google Scholar]
- Mendes, R.F.; Antunes, M.M.; Silva, P.; Barbosa, P.; Figueiredo, F.; Linden, A.; Rocha, J.; Valente, A.A.; Paz, F.A.A. A Lamellar Coordination Polymer with Remarkable Catalytic Activity. Chem. Eur. J. 2016, 22, 13136–13146. [Google Scholar] [CrossRef] [PubMed]
- Mirante, F.; Mendes, R.M.; Paz, F.A.A.; Balula, S.S. High Catalytic Efficiency of a Layered Coordination Polymer to Remove Simultaneous Sulfur and Nitrogen Compounds from Fuels. Catalysts 2020, 10, 731. [Google Scholar] [CrossRef]
- Win, D.T. Furfural—Gold from Garbage. Au J.Technol. 2005, 8, 185–190. [Google Scholar]
- Mamman, A.S.; Lee, J.-M.; Kim, Y.-C.; Hwang, I.T.; Park, N.-J.; Hwang, Y.K.; Chang, J.-S.; Hwang, J.-S. Furfural: Hemicellulose/xylosederived biochemical. Biofuel Bioprod. Bior. 2008, 2, 438–454. [Google Scholar]
- Research and Markets, Furfural and Furfuryl Alcohol: A Global Market Overview. 2018. Available online: https://www.researchandmarkets.com/reports/4622226/furfural-and-furfuryl-alcohol-a-global-market (accessed on 9 January 2021).
- Maldonado, G.M.G.; Assary, R.S.; Dumesic, J.A.; Curtiss, L.A. Acid-catalyzed conversion of furfuryl alcohol to ethyl levulinate in liquid ethanol. Energy Environ. Sci. 2012, 5, 8990–8997. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eerhart, A.J.J.E.; Patel, M.K.; Faaij, A.P.C. Fuels and plastics from lignocellulosic biomass via the furan pathway: An economic analysis. Biofuel Bioprod. Bior. 2015, 9, 307–325. [Google Scholar] [CrossRef]
- Haan, R.J.; Lange, J.-P. Gasoline Composition and Process for the Preparation of Alkylfurfurylether. U.S. Patent 8,372,164 B2, 12 February 2013. Available online: https://patentimages.storage.googleapis.com/c2/6f/90/cc012182280cd3/US8372164.pdf (accessed on 9 January 2021).
- Haan, J.R.; Lange, J.-P. Gasoline Composition and Process for the Preparationof Alkylfurfurylether. International Application Number: PCT/EP2008/067937. WO 2009/077606 A2, 25 June 2009. Available online: https://patentimages.storage.googleapis.com/7d/5d/a9/23f35e898d5267/WO2009077606A2.pdf (accessed on 9 January 2021).
- Bhansali, K.J.; Bhagat, P.R. Perylene supported metal free brønsted acid-functionalized porphyrin intertwined with benzimidazolium moiety for enhanced photocatalytic etherification of furfuryl alcohol. Fuel 2020, 278, 118394. [Google Scholar] [CrossRef]
- Paniagua, M.; Melero, J.A.; Iglesias, J.; Morales, G.; Hernández, B.; López-Aguado, C. Catalytic upgrading of furfuryl alcohol to bio-products: Catalysts screening and kinetic analysis. Appl. Catal. A Gen. 2017, 537, 74–82. [Google Scholar] [CrossRef]
- Vanderhaegen, B.; Neven, H.; Daenen, L.; Verstrepen, K.J.; Verachtert, H.; Derdelinckx, G.J. Furfuryl ethyl ether: Important aging flavor and a new marker for the storage conditions of beer. Agr. Food Chem. 2004, 52, 1661–1668. [Google Scholar] [CrossRef]
- Vanderhaegen, B.; Neven, H.; Coghe, S.; Verstrepen, K.J.; Verachtert, H.; Derdelinckx, G. Evolution of Chemical and Sensory Properties during Aging of Top-Fermented Beer. J. Agr. Food Chem. 2003, 51, 6782–6790. [Google Scholar] [CrossRef]
- Vanderhaegen, B.; Neven, H.; Verstrepen, K.J.; Delvaux, F.R.; Verachtert, H.; Derdelinckx, G.J. Influence of the Brewing Process on Furfuryl Ethyl Ether Formation during Beer Aging. Agr. Food Chem. 2004, 52, 6755–6764. [Google Scholar] [CrossRef]
- Herrmann, M.; Klotzbücher, B.; Wurzbacher, M.; Hanke, S.; Kattein, U.; Back, W.; Becker, T.; Krottenthaler, M.J.I. A New Validation of Relevant Substances for the Evaluation of Beer Aging Depending on the Employed Boiling System. Brewing 2010, 116, 41–48. [Google Scholar] [CrossRef]
- Harayama, K.; Hayase, F.; Kato, H. ontribution to Stale Flavor of 2-Furfuryl Ethyl Ether and Its Formation Mechanism in Beer. Biosci. Biotech. Bioch. 1995, 59, 1144–1146. [Google Scholar] [CrossRef] [Green Version]
- Spillman, P.J.; Pollnitz, A.P.; Liacopoulos, D.; Pardon, K.H.; Sefton, M.A. Formation and Degradation of Furfuryl Alcohol, 5-Methylfurfuryl Alcohol, Vanillyl Alcohol, and Their Ethyl Ethers in Barrel-Aged Wines. J. Agr. Food Chem. 1998, 46, 657–663. [Google Scholar] [CrossRef] [PubMed]
- Johannes, G.; Gruter, M. S-substituted 2-(alkoxymethyl)furans. U.S. Patent 8,231,693 B2, 31 July 2012. Available online: https://patentimages.storage.googleapis.com/43/72/8d/7aa4856861283b/US8231693.pdf (accessed on 9 January 2021).
- Patil, C.R.; Rode, C.V. Selective Production of Furanic Ethers from Lignocellulosic Biomass over Mesoporous Zr-Incorporated SBA-15 Catalyst. ChemistrySelect 2018, 3, 12504–12511. [Google Scholar]
- Eerhart, A.J.J.E.; Huijgen, W.J.J.; Grisel, R.J.H.; van der Waal, J.C.; de Jong, E.; de Dias, A.S.; Faaij, A.P.C.; Patel, M.K. Fuels and plastics from lignocellulosic biomass via the furan pathway; a technical analysis. RSC Adv. 2014, 4, 3536–3549. [Google Scholar] [CrossRef]
- Mulik, N.L.; Niphadkar, P.S.; Bokade, V. Synthesis of ethyl furfuryl ether (potential biofuel) by etherification of furfuryl alcohol with ethanol over heterogenized reusable H1Cs2PW12O40 catalyst. Res. Chem. Intermediat. 2020, 46, 2309–2325. [Google Scholar] [CrossRef]
- Chaffey, D.R.; Davies, T.E.; Taylor, S.H.; Graham, A.E. Etherification Reactions of Furfuryl Alcohol in the Presence of Orthoesters and Ketals: Application to the Synthesis of Furfuryl Ether Biofuels. ACS Sustain. Chem. Eng. 2018, 6, 4996–5002. [Google Scholar] [CrossRef]
- Hu, D.; Hu, H.; Jin, H.; Zhang, P.; Hu, Y.; Ying, S.; Li, X.; Yang, Y.; Zhang, J.; Wang, L. Building hierarchical zeolite structure by post-synthesis treatment to promote the conversion of furanic molecules into biofuels. Appl. Catal. A Gen. 2020, 590, 117338. [Google Scholar] [CrossRef]
- Tian, M.; McCormick, R.L.; Luecke, J.; de Jong, E.; van der Waal, J.C.; van Klink, G.P.M.; Boot, M.D. Anti-knock quality of sugar derived levulinic esters and cyclic ether. Fuel 2017, 202, 414–425. [Google Scholar] [CrossRef]
- De Jong, E.; Vijlbrief, T.; Hijkoop, R.; Gruter, J.-G.M.; van der Waal, J.C. Promising results with YXY Diesel components in an ESC test cycle using a PACCAR Diesel engine. Biomass Bioenerg. 2012, 36, 151–159. [Google Scholar] [CrossRef]
- Silva, J.F.L.; Grekin, R.; Mariano, A.P.; Filho, R.M. Making Levulinic Acid and Ethyl Levulinate Economically Viable: A Worldwide Technoeconomic and Environmental Assessment of Possible Routes. Energy Technol. Ger. 2018, 6, 613–639. [Google Scholar] [CrossRef]
- Ahmad, E.; Alam, M.I.; Pant, K.K.; Haider, M.A. Catalytic and mechanistic insights into the production of ethyl levulinate from biorenewable feedstocks. Green Chem. 2016, 18, 4804–4823. [Google Scholar] [CrossRef]
- Grand View Research, Ethyl Levulinate Market Size, Share and Trends Analysis Report by Application (Flavors, Fragrance), by Region (North America, Europe, Asia Pacific, Middle East and Africa, Central and South America) and Segment Forecast, 2015–2022. 2016. Available online: https://www.grandviewresearch.com/industry-analysis/ethyl-levulinate-market (accessed on 9 January 2021).
- Lange, J.-P.; van der Heide, E.; van Buijtenen, J.; Price, R. Furfural—A Promising Platform for Lignocellulosic Biofuels. ChemSusChem 2012, 5, 150–166. [Google Scholar] [CrossRef]
- Gupta, S.S.R.; Kantam, M.L. Catalytic conversion of furfuryl alcohol or levulinic acid into alkyl levulinates using a sulfonic acid-functionalized hafnium-based MOF. Catal. Commun. 2019, 124, 62–66. [Google Scholar] [CrossRef]
- Liu, X.; Pan, H.; Zhang, H.; Li, H.; Yang, S. Efficient Catalytic Upgradation of Bio-Based Furfuryl Alcohol to Ethyl Levulinate Using Mesoporous Acidic MIL-101(Cr). ACS Omega 2019, 4, 8390–8399. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.-F.; Li, H.; Zhang, H.; Pan, H.; Huang, S.; Yang, K.-L.; Yang, S. Efficient conversion of furfuryl alcohol to ethyl levulinate with sulfonic acid-functionalized MIL-101(Cr). RSC Adv. 2016, 6, 90232–90238. [Google Scholar] [CrossRef]
- Kolb, M.; Wichmann, H.; Schröder, U. GC/MS-screening analyses of valuable products in the aqueous phase from microwave-assisted hydrothermal processing of Lemna minor. Sustain. Chem. Pharm. 2019, 13, 100165. [Google Scholar] [CrossRef]
- Vermeire, F.H.; Carstensen, H.-H.; Herbinet, O.; Battin-Leclerc, F.; Marin, G.B.; Van Geem, K.M. The thermal decomposition of furfural: Molecular chemistry unraveled. P. Combust. Inst. 2019, 37, 445–452. [Google Scholar] [CrossRef] [Green Version]
- Furfuryl Alcohol Market Size, Share & Trends Analysis Report By Application (Resins, Solvent, Corrosion Inhibitor), By End Use (Foundry, Agriculture), By Region, And Segment Forecasts, 2020–2027. 2020. Available online: https://www.grandviewresearch.com/industry-analysis/furfuryl-alcohol-market (accessed on 9 January 2021).
- Tiong, Y.W.; Yap, C.L.; Gan, S.; Yap, W.S.P. Conversion of Biomass and Its Derivatives to Levulinic Acid and Levulinate Esters via Ionic Liquids. Ind. Eng. Chem. Res. 2018, 57, 4749–4766. [Google Scholar] [CrossRef]
- Kołodyńska, D.; Gęca, M.; Siek, M.; Hubicki, Z. Nitrilotris(methylenephosphonic) acid as a complexing agent in sorption of heavy metal ions on ion exchangers. Chem. Eng. J. 2013, 215, 948–958. [Google Scholar] [CrossRef]
- Vilela, S.M.F.; Ananias, D.; Fernandes, J.A.; Silva, P.; Gomes, A.C.; Silva, N.J.O.; Rodrigues, M.O.; Tomé, J.P.C.; Valente, A.A.; Ribeiro-Claro, P.; et al. Multifunctional micro- and nanosized metal–organic frameworks assembled from bisphosphonates and lanthanides. J. Mater. Chem. C 2014, 2, 3311–3327. [Google Scholar] [CrossRef]
- Monteiro, B.; Fernandes, J.A.; Pereira, C.C.L.; Vilela, S.M.F.; Tome, J.P.C.; Marcalo, J.; Paz, F.A.A. Metal-organic frameworks based on uranyl and phosphonate ligands. Acta Crystallogr. B 2014, 70, 28–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nanda, M.R.; Yuan, Z.; Qin, W.; Ghaziaskar, H.S.; Poirier, M.-A.; Xu, C.C. Thermodynamic and kinetic studies of a catalytic process to convert glycerol into solketal as an oxygenated fuel additive. Fuel 2014, 117, 470–477. [Google Scholar] [CrossRef]
- Song, D.; An, S.; Sun, Y.; Guo, Y. Efficient conversion of levulinic acid or furfuryl alcohol into alkyl levulinates catalyzed by heteropoly acid and ZrO2 bifunctionalized organosilica nanotubes. J. Catal. 2016, 333, 184–199. [Google Scholar] [CrossRef]
- Guo, H.; Abe, Y.; Qi, X.; Smith, R.L., Jr. Bifunctional carbon Ni/NiO nanofiber catalyst based on 5-sulfosalicylic acid for conversion of C5/C6 carbohydrates into ethyl levulinate. React. Chem. Eng. 2020, 5, 1759–1767. [Google Scholar] [CrossRef]
- Zaccheria, F.; Scotti, N.; Ravasio, N. Solid Acids for the Reaction of Bioderived Alcohols into Ethers for Fuel Applications. Catalysts 2019, 9, 172. [Google Scholar] [CrossRef] [Green Version]
- Zhao, D.; Prinsen, P.; Wang, Y.; Ouyang, W.; Delbecq, F.; Len, C.; Luque, R. Continuous Flow Alcoholysis of Furfuryl Alcohol to Alkyl Levulinates Using Zeolites. ACS Sustain. Chem. Eng. 2018, 6, 6901–6909. [Google Scholar] [CrossRef]
- Islam, M.M.; Bhunia, S.; Molla, R.A.; Bhaumik, A.; Islam, S.M. Organic Solid Acid Catalyst for Efficient Conversion of Furfuryl Alcohol to Biofuels. ChemistrySelect 2016, 1, 6079–6085. [Google Scholar] [CrossRef]
- Zhai, P.; Lv, G.; Cai, Z.; Zhu, Y.; Li, H.; Zhang, X.; Wang, F. Efficient Production of Ethyl Levulinate from Furfuryl Alcohol Catalyzed by Modified Zirconium Phosphate. ChemistrySelect 2019, 4, 3940–3947. [Google Scholar] [CrossRef]
- Zhou, H.; Song, J.; Kang, X.; Hu, J.; Yang, Y.; Fan, H.; Meng, Q.; Han, B. One-pot conversion of carbohydrates into gamma-valerolactone catalyzed by highly cross-linked ionic liquid polymer and Co/TiO2. RSC Adv. 2015, 5, 15267–15273. [Google Scholar] [CrossRef]
- Zhao, G.; Liu, M.; Xia, X.; Li, L.; Xu, B. Conversion of Furfuryl Alcohol into Ethyl Levulinate over Glucose-Derived Carbon-Based Solid Acid in Ethanol. Molecules 2019, 24, 1881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, H.; Shao, Y.; Liang, C.; Xu, Q.; Zhang, L.; Zhang, S.; Liu, S.; Hu, X. Sulfated attapulgite for catalyzing the conversion of furfuryl alcohol to ethyl levulinate: Impacts of sulfonation on structural transformation and evolution of acidic sites on the catalyst. Renew. Energ. 2020, 162, 1576–1586. [Google Scholar] [CrossRef]
- Shao, Y.; Du, W.; Gao, Z.; Sun, K.; Zhang, Z.; Li, Q.; Zhang, L.; Zhang, S.; Liu, Q.; Hu, X.J. Sulfated TiO2 nanosheets catalyzing conversion of biomass derivatives: Influences of the sulfation on distribution of Brønsted and Lewis acidic sites. Chem. Technol. Biot. 2020, 95, 1337–1347. [Google Scholar] [CrossRef]
- Zhao, G.; Hu, L.; Sun, Y.; Zeng, X.; Lin, L. Conversion of Biomass-Derived Furfuryl Alcohol into Ethyl Levulinate Catalyzed by Solid Acid in Ethanol. BioResources 2014, 9, 2634–2644. [Google Scholar] [CrossRef] [Green Version]
- Tiwari, M.S.; Gawade, A.B.; Yadav, G.D. Magnetically separable sulfated zirconia as highly active acidic catalysts for selective synthesis of ethyl levulinate from furfuryl alcohol. Green Chem. 2017, 19, 963–976. [Google Scholar]
- Zhang, Z.; Yuan, H.; Wang, Y.; Ke, Y.J. Preparation and characterisation of ordered mesoporous SO42−/Al2O3 and its catalytic activity in the conversion of furfuryl alcohol to ethyl levulinate. Solid State Chem. 2019, 280, 120991. [Google Scholar] [CrossRef]
- Shao, Y.; Li, Y.; Sun, K.; Zhang, Z.; Tian, H.; Gao, G.; Li, Q.; Liu, Q.; Liu, Q.; Hu, X. Sulfated Zirconia with Different Crystal Phases for the Production of Ethyl Levulinate and 5-Hydroxymethylfurfural. Energy Technol. Ger. 2020, 8, 1900951. [Google Scholar] [CrossRef]
- Topolyuk, Y.A.; Nekhaev, A.I. Functionalized nanocarbon materials as catalysts for the ethanolysis of furfuryl alcohol. Mendeleev Commun. 2018, 28, 93–95. [Google Scholar] [CrossRef]
- Guo, H.; Hirosaki, Y.; Qi, X.; Smith, R.L. Synthesis of ethyl levulinate over amino-sulfonated functional carbon materials. Renew. Energ. 2020, 157, 951–958. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, D.; Triantafyllidis, K.S.; Ouyang, W.; Luque, R.; Len, C. Microwave-assisted catalytic upgrading of bio-based furfuryl alcohol to alkyl levulinate over commercial non-metal activated carbon. Mol. Catal. 2020, 480, 110630. [Google Scholar] [CrossRef]
- Wu, J.; Shao, Y.; Jing, G.; Zhang, Z.; Ye, Z.; Hu, X.J. Design of graphene oxide by a one-pot synthetic route for catalytic conversion of furfural alcohol to ethyl levulinate. Chem. Technol. Biotechnol. 2019, 94, 3093–3101. [Google Scholar] [CrossRef]
- Zhu, S.; Chen, C.; Xue, Y.; Wu, J.; Wang, J.; Fan, W. Graphene Oxide: An Efficient Acid Catalyst for Alcoholysis and Esterification Reactions. ChemCatChem 2014, 6, 3080–3083. [Google Scholar] [CrossRef]
- Russo, P.A.; Antunes, M.M.; Neves, P.; Wiper, P.V.; Fazio, E.; Neri, F.; Barreca, F.; Mafra, L.; Pillinger, M.; Pinna, N.; et al. Mesoporous carbon–silica solid acid catalysts for producing useful bio-products within the sugar-platform of biorefineries. Green Chem. 2014, 16, 4292–4305. [Google Scholar]
- Russo, P.A.; Antunes, M.M.; Neves, P.; Wiper, V.P.; Fazio, E.; Neri, F.; Barreca, F.; Mafra, L.; Pillinger, M.; Pinna, N.; et al. Solid acids with SO3H groups and tunable surface properties: Versatile catalysts for biomass conversion. J. Mater. Chem. A 2014, 2, 11813–11824. [Google Scholar] [CrossRef] [Green Version]
- Gao, X.; Peng, L.; Li, H.; Chen, K. Formation of Humin and Alkyl Levulinate in the Acid-catalyzed Conversion of Biomass-derived Furfuryl Alcohol. BioResources 2015, 10, 6548–6564. [Google Scholar]
- Onkarappa, S.B.; Bhat, N.S.; Dutta, S. Preparation of alkyl levulinates from biomass-derived 5-(halomethyl)furfural (X = Cl, Br), furfuryl alcohol, and angelica lactone using silica-supported perchloric acid as a heterogeneous acid catalyst. Biomass Convers. Biorefin. 2020, 10, 849–856. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, P.; Wu, Z.; Yue, C.; Wei, X.; Zheng, J.; Xiang, M.; Liu, B. Efficient synthesis of niobium pentoxide nanowires and application in ethanolysis of furfuryl alcohol. RSC Adv. 2020, 10, 5690–5696. [Google Scholar]
- Skrodczky, K.; Antunes, M.M.; Han, X.; Santangelo, S.; Scholz, G.; Valente, A.A.; Pinna, N.; Russo, P.A. Niobium pentoxide nanomaterials with distorted structures as efficient acid catalysts. Commun. Chem. 2019, 2, 129. [Google Scholar] [CrossRef]
- Ren, D.; Fu, J.; Li, L.; Liu, Y.; Jin, F.; Huo, Z. Efficient conversion of biomass-derived furfuryl alcohol to levulinate esters over commercial α-Fe2O3. RSC Adv. 2016, 6, 22174–22178. [Google Scholar] [CrossRef]
- Chada, R.R.; Koppadi, K.S.; Enumula, S.S.; Kondeboina, M.; Kamaraju, S.R.R.; Burri, D.R. Effect of WOx Doping into Pt/SiO2 Catalysts for Glycerol Hydrogenolysis to 1,3-Propanediol in Liquid Phase. Catal. Lett. 2018, 148, 1731–1738. [Google Scholar] [CrossRef]
- Lingaiah, N. One pot selective transformation of biomass derived chemicals towards alkyl levulinates over titanium exchanged heteropoly tungstate catalysts. Catal. Today 2018, 309, 269–275. [Google Scholar]
- Neves, P.; Russo, P.A.; Fernandes, A.; Antunes, M.M.; Farinha, J.; Pillinger, M.; Ribeiro, M.F.; Castanheiro, J.E.; Valente, A.A. Mesoporous zirconia-based mixed oxides as versatile acid catalysts for producing bio-additives from furfuryl alcohol and glycerol. Appl. Catal. A Gen. 2014, 487, 148–157. [Google Scholar] [CrossRef] [Green Version]
- Guo, Q.; Yang, F.; Liu, X.; Sun, M.; Guo, Y.; Wang, Y. Low-cost synthesis of nanoaggregate SAPO-34 and its application in the catalytic alcoholysis of furfuryl alcohol. Chin. J. Catal. 2020, 41, 1772–1781. [Google Scholar] [CrossRef]
- Wang, M.-Y.; Su, H.; Zhai, G.-Y.; Yu, Q.-Y.; Wang, H.-H.; Jiang, Z.-D.; Li, X.-H.; Chen, J.-S. Synergy of B and Al Dopants in Mesoporous MFI Nanocrystals for Highly Selective Alcoholysis of Furfuryl Alcohol into Ethyl Levulinate. Energy Technol. Ger. 2019, 7, 1900271. [Google Scholar] [CrossRef]
- Nandiwale, K.Y.; Pande, A.M.; Bokade, V.V. One step synthesis of ethyl levulinate biofuel by ethanolysis of renewable furfuryl alcohol over hierarchical zeolite catalyst. RSC Adv. 2015, 5, 79224–79231. [Google Scholar] [CrossRef]
- Lange, J.-P.; van de Graaf, W.D.; Haan, R.J. Conversion of Furfuryl Alcohol into Ethyl Levulinate using Solid Acid Catalysts. ChemSusChem 2009, 2, 437–441. [Google Scholar] [CrossRef]
- Cao, Q.; Guan, J.; Peng, G.; Hou, T.; Zhou, J.; Mu, X. Solid acid-catalyzed conversion of furfuryl alcohol to alkyl tetrahydrofurfuryl ether. Catal. Commun. 2015, 58, 76–79. [Google Scholar] [CrossRef]
- Enumula, S.S.; Koppadi, K.S.; Gurram, V.R.B.; Burri, D.R.; Kamaraju, S.R.R. Conversion of furfuryl alcohol to alkyl levulinate fuel additives over Al2O3/SBA-15 catalyst. Sustain. Energy Fuels 2017, 1, 644–651. [Google Scholar] [CrossRef]
- Neves, P.; Lima, S.; Pillinger, M.; Rocha, S.M.; Rocha, J.; Valente, A.A. Conversion of furfuryl alcohol to ethyl levulinate using porous aluminosilicate acid catalysts. Catal. Today 2013, 218, 76–84. [Google Scholar] [CrossRef]
- Neves, P.; Antunes, M.M.; Russo, P.A.; Abrantes, J.P.; Lima, S.; Fernandes, A.; Pillinger, M.; Rocha, S.M.; Ribeiro, M.F.; Valente, A.A. Production of biomass-derived furanic ethers and levulinate esters using heterogeneous acid catalysts. Green Chem. 2013, 15, 3367–3376. [Google Scholar] [CrossRef]
- Nandiwale, K.Y.; Pande, A.M.; Bokade, V.V. HPW anchored Meso-HZ-5, a novel catalyst for selective synthesis of ethyl levulinate biofuel by alcoholysis of biomass-derived furfuryl alcohol. Environ. Prog. Sustain. 2018, 37, 1736–1742. [Google Scholar] [CrossRef]
- Kong, X.; Zhang, X.; Han, C.; Li, C.; Yu, L.; Liu, J. Ethanolysis of biomass based furfuryl alcohol to ethyl levulinate over Fe modified USY catalyst. Mol. Catal. 2017, 443, 186–192. [Google Scholar] [CrossRef]
- Song, D.; An, S.; Sun, Y.; Zhang, P.; Guo, Y.; Zhou, D. Ethane-Bridged Organosilica Nanotubes Functionalized with Arenesulfonic Acid and Phenyl Groups for the Efficient Conversion of Levulinic Acid or Furfuryl Alcohol to Ethyl Levulinate. ChemCatChem 2016, 8, 2037–2048. [Google Scholar] [CrossRef]
- Song, D.; An, S.; Lu, B.; Guo, Y.; Leng, J. Arylsulfonic acid functionalized hollow mesoporous carbon spheres for efficient conversion of levulinic acid or furfuryl alcohol to ethyl levulinate. Appl. Catal. B Environ. 2015, 179, 445–457. [Google Scholar]
- An, S.; Song, D.; Lu, B.; Yang, X.; Guo, Y.H. Morphology Tailoring of Sulfonic Acid Functionalized Organosilica Nanohybrids for the Synthesis of Biomass-Derived Alkyl Levulinates. Chem. Eur. J. 2015, 21, 10786–10798. [Google Scholar] [CrossRef]












| Entry | T (°C) | Gly:Bza Molar Ratio | Conv. (%) | Product Yield (%) | ||
|---|---|---|---|---|---|---|
Dioxolane | Dioxane | Total Acetals | ||||
| 1 | 90 | 1:3 | 73 | 18 | 52 | 70 |
| 2 | 90 | 1:2 | 99 | 24 | 73 | 97 |
| 3 | 120 | 1:3 | 100 | 28 | 65 | 93 |
| 4 | 120 | 1:2 | 98 | 28 | 66 | 94 |
| Entry | Catalyst 2 (Solvent) | T (°C) | Gly:Bza (mol) | Cat:Gly (wt) | t (h) | Conv. (%) | Dioxolane Yield (%) | Dioxane Yield (%) | Total Yield (%) | Ref |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1 | 50 | 1:2 | 0.01 | 0.5/2 | 10/53 | 2/18 | 4/34 | 6/52 | - |
| 2 | 1 | 50 | 1:2 | 0.1 | 0.5/2 | 38/77 | 12/52 | 24/21 | 38/73 | - |
| 3 | 1 | 70 | 1:2 | 0.01 | 0.5/2 | 46/73 | 14/19 | 30/51 | 44/70 | - |
| 4 | 1 | 70 | 1:2 | 0.1 | 0.5/2 | 67/82 | 19/21 | 45/61 | 64/82 | - |
| 5 | 1 | 90 | 1:2 | 0.01 | 0.5/2 | 58/77 | 17/22 | 41/53 | 58/75 | - |
| 6 | 1 | 90 | 1:2 | 0.1 | 0.5/2 | 80/91 | 22/26 | 54/64 | 76/90 | - |
| 7 | Resin IRA-120 | 90 | 1:2 | 0.1 | 2 | 85 | nm | nm | 51 | [110] |
| 8 | Amberlyst-36 (chloroform) | 59 | 1.1:1 | 0.01 | 4 | nm | 56 | 44 | 94 | [79] |
| 9 | Amberlyst-15 (toluene) | 70 | 1:1.1 | 0.06 | 4 | nm | nm | nm | 70 | [77] |
| 10 | MoOx-TiO2-ZrO2 | 100 | 1:1 | 0.01 | 0.5 | 32 | 15 | 17 | 32 | [111] |
| 11 | MoO3/SiO2 (toluene) | 100 | 1.1:1 | 0.1 | 8 | 72 | 29 | 43 | 72 | [93] |
| 12 | Meso-SnO2-T350 | 100 | 1:1 | 0.1 | 0.5 | 60 | nm | 30 | 30 | [82] |
| 13 | SO42−/SnO2 | 100 | 1:1 | 0.05 | 0.5 | 80 | 32 | 48 | 80 | [33] |
| 14 | Beta (Si/Al = 25) (chloroform) | 59 | 1.1:1 | 0.1 | 6 | nm | 41 | 59 | 94 | [79] |
| 15 | Beta (Si/Al = 25) | 100 | 1:1 | 0.1 | 0.5 | 60 | nm | 29 | 29 | [82] |
| 16 | W-Beta (Si/Al = 10) | 30 | 1:1 | 0.05 | 1 | 95 | 74 | 21 | 95 | [19] |
| 17 | Hf-SBA-15 (t-butanol) | 90 | 1:1 | 1.09 | 6 | 63 | 15 | 25 | 40 | [83] |
| 18 | Al-SBA-15 | 100 | 1:1 | 0.005 | 8 | 72 | 60 | 12 | 72 | [32] |
| 19 | Al-SBA-15 | 100 | 1:1 | 1.09 | 8 | 82 | 68 | 14 | 82 | [31] |
| 20 | 30SiW12/ MCM-41 | 30 | 1:1.2 | 0.11 | 1 | 91 | 68 | 23 | 91 | [88] |
| 21 | 40TSA/ MCM-48 | 30 | 1:2 | 0.05 | 1 | 99 | 34 | 65 | 99 | [113] |
| 22 | POSS-SO3H | 30 | 1:1 | 0.018 | 2 | 90 | 70 | 20 | 90 | [80] |
| 23 | RHASO3H (toluene) | 120 | 1:2 | 0.01 | 8 | 62 | nm | nm | 62 | [112] |
| 24 | An-POP-SO3H | 40 | 1:1 | 0.217 | 1.5 | 78 | 51 | 24 | 75 | [84] |
| 25 | RHABIm-HSO4 | 120 | 1:2 | 0.005 | 6 | 54 | 46 | 8 | 54 | [102] |
| 26 | 6BBnU/6 | 60 | 2:1 | 0.05 | 1 | 84 | 50 | 34 | 84 | [86] |
| Entry | Catalyst 2 (Solvent) | T (°C) | Gly:Fur (mol) | Cat:Gly (wt) | t (h) | Conv. (%) | Dioxolane Yield (%)  | Dioxane Yield (%)  | Total Acetals Yield (%) | Ref |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1 | 50 | 1:2 | 0.01 | 0.5/2/4 | 37/57/62 | 24/34/36 | 13/22/25 | 37/56/61 | - |
| 2 | 1 | 50 | 1:2 | 0.1 | 0.5/2/4 | 48/62/74 | 26/36/43 | 22/25/30 | 48/61/73 | - |
| 3 | 1 | 70 | 1:2 | 0.01 | 0.5/2/4 | 43/59/66 | 33/45/46 | 9/13/16 | 42/58/62 | - |
| 4 | 1 | 70 | 1:2 | 0.1 | 0.5/2/4 | 57/68/81 | 35/44/52 | 21/22/29 | 56/66/81 | - |
| 5 | 1 | 90 | 1:2 | 0.01 | 0.5/2/4 | 61/71/80 | 42/51/63 | 19/20/15 | 62/71/78 | - |
| 6 | 1 | 90 | 1:2 | 0.1 | 0.5/2/4 | 70/79/93 | 47/52/64 | 21/24/27 | 68/76/91 | - |
| 7 | Al-SBA-15 | 100 | 1:1 | 1.09 | 12 | 74 | 50 | 24 | 74 | [31,32] |
| 8 | Fe/Al-SBA-15 | 100 | 1:1.5 | 0.54 | 12 | 100 | 60 | 40 | 100 | [32] |
| 9 | Zr-Mont | Rt | 1:1 | 0.10 | 4 | 84 | 52 | 26 | 78 | [106] |
| 10 | 6BBNu/6 | 60 | 2:1 | 0.05 | 1 | 69 | 37 | 32 | 69 | [86] |
| 11 | ReHectMw | 40 | 1:1 | 0.05 | 4 | 32 | 12 | 11 | 23 | [101] |
| 12 | MK-10-SC | 40 | 1:1 | 0.05 | 4 | 62 | 47 | 7 | 54 | [101] |
| 13 | SBA-15-SC | 40 | 1:1 | 0.05 | 4 | 74 | 38 | 4 | 42 | [101] |
| 14 | Al-MCM-41 | 100 | 1:5 | 0.1 | 2 | nm | nm | nm | 80 | [114] |
| 15 | TSA-nMCM-48 | 30 | 1:2 | 0.02 | 0.67 | 87 | 37 | 50 | 87 | [113] |
| 16 | H3PW12O40/ MCM-41 (toluene) | >110 c | 1:1.05 | 0.05 | 1.67 | nm | nm | nm | 89 | [115] |
| 17 | MoO3/SnO2 | rt | 1: 1 | 0.05 | 0.5 | 75 | 45 | 26 | 71 | [81] |
| 18 | WO3/SnO2 | rt | 1:1 | 0.05 | 0.5 | 67 | 40 | 22 | 62 | [81] |
| 19 | SO42−/SnO2 | rt | 1:1 | 0.05 | 0.5 | 82 | 55 | 27 | 82 | [78] |
| 20 | An-POP-SO3H | 40 | 1:1 | 0.2 | 1.5 | 85 | 64 | 22 | 85 | [84] |
| 21 | Amberlyst-15 (cyclohexane) | 70 | 1:1.1 | 0.06 | 4 | nm | nm | nm | 80 | [77] |
| 22 | Amberlite-IR120 | rt | 1:1 | 0.10 | 4 | 91 | 37 | 19 | 56 | [106] |
| 23 | 80LS20PS450H+ | 100 | 1:2 | 0.005 | 1 | 93 | 47 | 46 | 93 | [103] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antunes, M.M.; Mendes, R.F.; Paz, F.A.A.; Valente, A.A. Versatile Coordination Polymer Catalyst for Acid Reactions Involving Biobased Heterocyclic Chemicals. Catalysts 2021, 11, 190. https://doi.org/10.3390/catal11020190
Antunes MM, Mendes RF, Paz FAA, Valente AA. Versatile Coordination Polymer Catalyst for Acid Reactions Involving Biobased Heterocyclic Chemicals. Catalysts. 2021; 11(2):190. https://doi.org/10.3390/catal11020190
Chicago/Turabian StyleAntunes, Margarida M., Ricardo F. Mendes, Filipe A. Almeida Paz, and Anabela A. Valente. 2021. "Versatile Coordination Polymer Catalyst for Acid Reactions Involving Biobased Heterocyclic Chemicals" Catalysts 11, no. 2: 190. https://doi.org/10.3390/catal11020190