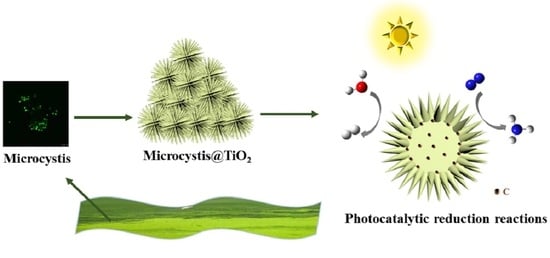
Microcystis@TiO2 Nanoparticles for Photocatalytic Reduction Reactions: Nitrogen Fixation and Hydrogen Evolution
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of Photocatalysts
2.2. Photocatalytic Activity
2.2.1. Photocatalytic Nitrogen Fixation Performance of Microcystis@TiO2 Composites
2.2.2. Photocatalytic Hydrogen Generation Performance of Microcystis@TiO2 Composites
2.3. Photoelectrochemical Properties
2.4. Mechanism
3. Materials and Methods
3.1. Synthesis of Microcystis@TiO2 Composites
3.2. Characterization
3.3. Photocatalytic Nitrogen Fixation
3.4. Photocatalytic Hydrogen Evolution
3.5. Photoelectrochemical Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xiang, Q.; Li, F.; Zhang, D.; Liao, Y.; Zhou, H. Plasma-based surface modification of g-C3N4 nanosheets for highly efficient photocatalytic hydrogen evolution. Appl. Surf. Sci. 2019, 495, 143520. [Google Scholar] [CrossRef]
- Xiang, Q.; Cheng, F.; Lang, D. Hierarchical layered WS2/graphene-modified CdS nanorods for efficient photocatalytic hdrogen evolution. Chemsuschem 2016, 9, 996–1002. [Google Scholar] [CrossRef]
- Xiang, Q.; Yu, J. Graphene-based photocatalysts for hydrogen generation. J. Phys. Chem. Lett. 2013, 4, 753–759. [Google Scholar] [CrossRef]
- Cheng, L.; Li, X.; Zhang, H.; Xiang, Q. Two-dimensional transition metal MXene-based photocatalysts for solar fuel generation. J. Phys. Chem. Lett. 2019, 10, 3488–3494. [Google Scholar] [CrossRef]
- Shen, R.; Ren, D.; Ding, Y.; Guan, Y.; Ng, Y.H.; Zhang, P.; Li, X. Nanostructured CdS for efficient photocatalytic H2 evolution: A review. Sci. China Mater. 2020, 63, 2153–2188. [Google Scholar] [CrossRef]
- Xiao, L.; Li, X.; Zhang, J.; He, Z. MgB4 MXene-like nanosheets for photocatalytic hydrogen evolution. ACS Appl. Nano Mater. 2021, 4, 12779–12787. [Google Scholar] [CrossRef]
- Jiang, Z.; Chen, Q.; Zheng, Q.; Shen, R.; Zhang, P.; Li, X. Constructing 1D/2D schottky-based heterojunctions between Mn0.2Cd0.8S nanorods and Ti3C2 nanosheets for boosted photocatalytic H2 evolution. Acta Phys.-Chim. Sin. 2021, 37, 2010059. [Google Scholar]
- Wageh, S.; Al-Ghamdi, A.A.; Jafer, R.; Li, X.; Zhang, P. A new heterojunction in photocatalysis: S-scheme heterojunction. Chin. J. Catal. 2021, 42, 667–669. [Google Scholar] [CrossRef]
- Shen, R.; Ding, Y.; Li, S.; Zhang, P.; Xiang, Q.; Ng, Y.H.; Li, X. Constructing low-cost Ni3C/twin-crystal Zn0.5Cd0.5S heterojunction/homojunction nanohybrids for efficient photocatalytic H2 evolution. Chin. J. Catal. 2021, 42, 25–36. [Google Scholar] [CrossRef]
- He, Z.; Zhang, J.; Li, X.; Guan, S.; Dai, M.; Wang, S. 1D/2D Heterostructured photocatalysts: From design and unique properties to their environmental applications. Small 2020, 16, 2005051. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.J.; He, Z.L.; Xu, S.S.; Li, X.; Zhang, J.; Zhan, X.P.; Dai, M.; Wang, S.G. In situ liquid-phase growth strategies of g-C3N4 solar-driven heterogeneous catalysts for environmental applications. Sol. RRL 2021, 5, 2100233. [Google Scholar] [CrossRef]
- Shi, R.; Zhang, X.; Waterhouse, G.I.N.; Zhao, Y.; Zhang, T. The journey toward low temperature, low pressure catalytic nitrogen fixation. Adv. Energy Mater. 2020, 10, 2000659. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.Y.; Ichihara, F.; Pang, H.; Chen, H.; Ye, J.H. Nitrogen fixation reaction derived from nanostructured catalytic materials. Adv. Funct. Mater. 2018, 28, 1803309. [Google Scholar] [CrossRef]
- Shen, R.; Lu, X.; Zheng, Q.; Chen, Q.; Ng, Y.H.; Zhang, P.; Li, X. Tracking S-scheme charge transfer pathways in Mo2C/CdS H2 evolution photocatalysts. Sol. RRL 2021, 5, 2100177. [Google Scholar] [CrossRef]
- Lan, Z.A.; Ren, W.; Chen, X.; Zhang, Y.; Wang, X. Conjugated donor-acceptor polymer photocatalysts with electron-output ldquotentaclesrdquo for efficient hydrogen evolution. Appl. Catal. B 2019, 245, 596–603. [Google Scholar] [CrossRef]
- Lin, L.; Lin, Z.; Zhang, J.; Cai, X.; Lin, W.; Yu, Z.; Wang, X. Molecular-level insights on the reactive facet of carbon nitride single crystals photocatalysing overall water splitting. Nat. Catal. 2020, 3, 649–655. [Google Scholar] [CrossRef]
- Cheng, L.; Zhang, D.; Liao, Y.; Li, F.; Zhang, H.; Xiang, Q. Constructing functionalized plasmonic gold/titanium dioxide nanosheets with small gold nanoparticles for efficient photocatalytic hydrogen evolution. J. Colloid Interface Sci. 2019, 555, 94–103. [Google Scholar] [CrossRef]
- Li, Y.; Gong, F.; Zhou, Q.; Feng, X.; Fan, J.; Xiang, Q. Crystalline isotype heptazine-/triazine-based carbon nitride heterojunctions for an improved hydrogen evolution. Appl. Catal. B 2020, 268, 118381. [Google Scholar] [CrossRef]
- Yu, J.; Low, J.; Xiao, W.; Zhou, P.; Jaroniec, M. Enhanced photocatalytic CO2-reduction activity of anatase TiO2 by coexposed {001} and {101} Facets. J. Am. Chem. Soc. 2014, 136, 8839–8842. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.M.; Zhao, K.; Xiong, J.Y.; Wei, Y.; Han, C.; Li, W.J.; Cheng, G. A 1D/2D WO3 nanostructure coupled with a nanoparticulate CuO cocatalyst for enhancing solar-driven CO2 photoreduction: The impact of the crystal facet. Sustain. Energy Fuels 2020, 4, 2593–2603. [Google Scholar] [CrossRef]
- Li, S.; Wang, C.; Cai, M.; Yang, F.; Liu, Y.; Chen, J.; Zhang, P.; Li, X.; Chen, X. Facile fabrication of TaON/Bi2MoO6 core-shell S-scheme heterojunction nanofibers for boosting visible-light catalytic levofloxacin degradation and Cr(VI) reduction. Chem. Eng. J. 2022, 428, 131158. [Google Scholar] [CrossRef]
- He, Z.; Kim, C.; Lin, L.; Jeon, T.H.; Lin, S.; Wang, X.; Choi, W. Formation of heterostructures via direct growth CN on h-BN porous nanosheets for metal-free photocatalysis. Nano Energy 2017, 42, 58–68. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, D.; Feng, X.; Xiang, Q. Enhanced photocatalytic hydrogen production activity of highly crystalline carbon nitride synthesized by hydrochloric acid treatment. Chin. J. Catal. 2020, 41, 21–30. [Google Scholar] [CrossRef]
- Cheng, M.; Xiao, C.; Xie, Y. Photocatalytic nitrogen fixation: The role of defects in photocatalysts. J. Mater. Chem. A 2019, 7, 19616–19633. [Google Scholar] [CrossRef]
- Ran, Y.; Yu, X.; Liu, J.; Cui, J.; Wang, J.; Wang, L.; Zhang, Y.; Xiang, X.; Ye, J. Polymeric carbon nitride with frustrated Lewis pair sites for enhanced photofixation of nitrogen. J. Mater. Chem. A 2020, 8, 13292–13298. [Google Scholar] [CrossRef]
- He, Z.; Kim, C.; Jeon, T.H.; Choi, W. Hydrogenated heterojunction of boron nitride and titania enables the photocatalytic generation of H2 in the absence of noble metal catalysts. Appl. Catal. B 2018, 237, 772–782. [Google Scholar] [CrossRef]
- He, Z.L.; Que, W.X.; He, Y.C. Enhanced photocatalytic performance of sensitized mesoporous TiO2 nanoparticles by carbon mesostructures. RSC Adv. 2014, 4, 3332–3339. [Google Scholar] [CrossRef]
- He, Z.; Que, W.; Chen, J.; Yin, X.; He, Y.; Ren, J. Photocatalytic degradation of methyl orange over nitrogen-fluorine codoped TiO2 nanobelts prepared by solvothermal synthesis. ACS Appl. Mater. Inter. 2012, 4, 6816–6826. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.K.; Zhang, P.; Wan, D.Y.; Xue, C.; Zhao, J.T.; Shao, G.S. Direct evidence of 2D/1D heterojunction enhancement on photocatalytic activity through assembling MoS2 nanosheets onto super-long TiO2 nanofibers. Appl. Surf. Sci. 2020, 504, 144361. [Google Scholar] [CrossRef]
- Shu, Y.; Ji, J.; Zhou, M.; Liang, S.; Xie, Q.; Li, S.; Liu, B.; Deng, J.; Cao, J.; Liu, S.; et al. Selective photocatalytic oxidation of gaseous ammonia at ppb level over Pt and F modified TiO2. Appl. Catal. B 2022, 300, 120688. [Google Scholar] [CrossRef]
- Wanag, A.; Kusiak-Nejman, E.; Czyżewski, A.; Moszyński, D.; Morawski, A.W. Influence of rGO and preparation method on the physicochemical and photocatalytic properties of TiO2/reduced graphene oxide photocatalysts. Catalysts 2021, 11, 1333. [Google Scholar] [CrossRef]
- Diban, N.; Pacuła, A.; Kumakiri, I.; Barquín, C.; Rivero, M.J.; Urtiaga, A.; Ortiz, I. TiO2–Zeolite metal composites for photocatalytic degradation of organic pollutants in water. Catalysts 2021, 11, 1367. [Google Scholar] [CrossRef]
- Raditoiu, V.; Raditoiu, A.; Raduly, M.F.; Amariutei, V.; Gifu, I.C.; Anastasescu, M. Photocatalytic Behavior of Water-Based Styrene-Acrylic Coatings Containing TiO2 Sensitized with Metal-Phthalocyanine Tetracarboxylic Acids. Coatings 2017, 7, 229. [Google Scholar] [CrossRef] [Green Version]
- Bellè, U.; Pelizzari, F.; Lucotti, A.; Castiglioni, C.; Ormellese, M.; Pedeferri, M.; Diamanti, M.V. Immobilized Nano-TiO2 Photocatalysts for the Degradation of Three Organic Dyes in Single and Multi-Dye Solutions. Coatings 2020, 10, 919. [Google Scholar] [CrossRef]
- Yu, X.; Qiu, H.; Wang, Z.; Wang, B.; Meng, Q.; Sun, S.; Tang, Y.; Zhao, K. Constructing the Z-scheme TiO2/Au/BiOI nanocomposite for enhanced photocatalytic nitrogen fixation. Appl. Surf. Sci. 2021, 556, 149785. [Google Scholar] [CrossRef]
- Ding, Z.; Sun, M.; Liu, W.; Sun, W.; Meng, X.; Zheng, Y. Ultrasonically synthesized N-TiO2/Ti3C2 composites: Enhancing sonophotocatalytic activity for pollutant degradation and nitrogen fixation. Sep. Purif. Technol. 2021, 276, 119287. [Google Scholar] [CrossRef]
- Lan, K.; Wang, R.C.; Wei, Q.L.; Wang, Y.X.; Hong, A.; Feng, P.Y.; Zhao, D.Y. Stable Ti(3+) defects in oriented mesoporous titania frameworks for efficient photocatalysis. Angew. Chem. Int. Ed. 2020, 59, 17676–17683. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Meng, K.; Cheng, B.; Wang, S.; Xu, J.; Yu, J. Unique S-scheme heterojunctions in self-assembled TiO2/CsPbBr3 hybrids for CO2 photoreduction. Nat. Commun. 2020, 11, 4613. [Google Scholar] [CrossRef]
- Shan, K.; Wang, X.; Yang, H.; Zhou, B.; Song, L.; Shang, M. Use statistical machine learning to detect nutrient thresholds in Microcystis blooms and microcystin management. Harmful Algae 2020, 94, 101807. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Giesy, J.P.; Adamovsky, O.; Svirčev, Z.; Meriluoto, J.; Codd, G.A.; Mijovic, B.; Shi, T.; Tuo, X.; Li, S.C.; et al. Challenges of using blooms of Microcystis spp. in animal feeds: A comprehensive review of nutritional, toxicological and microbial health evaluation. Sci. Total Environ. 2021, 764, 142319. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Church, J.; Son, Y.; Kim, K.T.; Lee, W.H. Recent advances in ultrasonic treatment: Challenges and field applications for controlling harmful algal blooms (HABs). Ultrason. Sonochem. 2017, 38, 326–334. [Google Scholar] [CrossRef] [PubMed]
- Oehrle, S.; Rodriguez-Matos, M.; Cartamil, M.; Zavala, C.; Rein, K.S. Toxin composition of the 2016 Microcystis aeruginosa bloom in the St. Lucie Estuary, Florida. Toxicon 2017, 138, 169–172. [Google Scholar] [CrossRef] [PubMed]
- Sha, J.; Xiong, H.; Li, C.; Lu, Z.; Zhang, J.; Zhong, H.; Zhang, W.; Yan, B. Harmful algal blooms and their eco-environmental indication. Chemosphere 2021, 274, 129912. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Tao, Y.; Zhan, X.-M.; Dao, G.-H.; Hu, H.Y. UV-C irradiation for harmful algal blooms control: A literature review on effectiveness, mechanisms, influencing factors and facilities. Sci. Total Environ. 2020, 723, 137986. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Sun, P.; Zhang, J.; Esquivel-Elizondo, S.; Wu, Y. Microorganisms-based methods for harmful algal blooms control: A review. Bioresour. Technol. 2018, 248, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Jin, X.; Chen, X.; Wang, A.; Zhang, J.; Zhang, J.; Zhao, Z.; Gao, M.; Razzari, L.; Liu, H. A microorganism Bred TiO2/Au/TiO2 heterostructure for whispering gallery mode resonance assisted plasmonic photocatalysis. ACS Nano 2020, 14, 13876–13885. [Google Scholar] [CrossRef] [PubMed]
- Sutthiumporn, K.; Kawi, S. Promotional effect of alkaline earth over Ni–La2O3 catalyst for CO2 reforming of CH4: Role of surface oxygen species on H2 production and carbon suppression. Int. J. Hydrog. Energy 2011, 36, 14435–14446. [Google Scholar] [CrossRef]
- Mu, Q.; Sun, Y.; Guo, A.; Yu, X.; Xu, X.; Cai, A.; Wang, X. Bio-templated synthesis of Fe3O4–TiO2 composites derived from Chlorella pyrenoidosa with enhanced visible-light photocatalytic performance. Mater. Res. Express. 2019, 6, 950–953. [Google Scholar] [CrossRef]
- Liao, Y.; Qian, J.; Xie, G.; Han, Q.; Dang, W.; Wang, Y.; Lv, L.; Zhao, S.; Luo, L.; Zhang, W.; et al. 2D-layered Ti3C2 MXenes for promoted synthesis of NH3 on P25 photocatalysts. Appl. Catal. B 2020, 273, 119054. [Google Scholar] [CrossRef]











Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Chang, J.; Zhang, S.; Xiao, L.; Wu, X.; He, Z. Microcystis@TiO2 Nanoparticles for Photocatalytic Reduction Reactions: Nitrogen Fixation and Hydrogen Evolution. Catalysts 2021, 11, 1443. https://doi.org/10.3390/catal11121443
Li X, Chang J, Zhang S, Xiao L, Wu X, He Z. Microcystis@TiO2 Nanoparticles for Photocatalytic Reduction Reactions: Nitrogen Fixation and Hydrogen Evolution. Catalysts. 2021; 11(12):1443. https://doi.org/10.3390/catal11121443
Chicago/Turabian StyleLi, Xuan, Jingcai Chang, Shijie Zhang, Lihui Xiao, Xiaoge Wu, and Zuoli He. 2021. "Microcystis@TiO2 Nanoparticles for Photocatalytic Reduction Reactions: Nitrogen Fixation and Hydrogen Evolution" Catalysts 11, no. 12: 1443. https://doi.org/10.3390/catal11121443