Hybrid Structure of TiO2-Graphitic Carbon as a Support of Pt Nanoparticles for Catalyzing Oxygen Reduction Reaction
Abstract
:1. Introduction
2. Results and Discussion
2.1. Preparation and Characterization of the TiO2-SP Hybrid
2.2. Comparison of the Structures of SP Carbon and Vulcan XC-72
2.3. Characterization of the Pt-TiO2-SP Hybrid
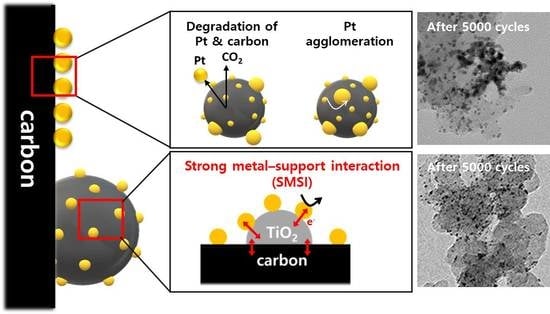
2.4. Electrochemical Characterization of Pt-TiO2-SP
3. Materials and Methods
3.1. Materials
3.2. Synthesis of TiO2-SP Carbon Composite
3.3. Characterization
3.4. Electrochemical Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Barbosa, E.C.; Parreira, L.S.; de Freitas, I.C.; Aveiro, L.R.; de Oliveira, D.C.; dos Santos, M.C.; Camargo, P.H. Pt-decorated TiO2 materials supported on carbon: Increasing activities and stabilities toward the ORR by tuning the Pt loading. ACS Appl. Energy Mater. 2019, 2, 5759–5768. [Google Scholar] [CrossRef]
- Dos Reis, F.V.; Antonin, V.S.; Hammer, P.; Santos, M.C.; Camargo, P.H. Carbon-supported TiO2–Au hybrids as catalysts for the electrogeneration of hydrogen peroxide: Investigating the effect of TiO2 shape. J. Catal. 2015, 326, 100–106. [Google Scholar] [CrossRef]
- Zana, A.; Rüdiger, C.; Kunze-Liebhäuser, J.; Granozzi, G.; Reeler, N.E.; Vosch, T.; Kirkensgaard, J.J.; Arenz, M. Core-shell TiO2@ C: Towards alternative supports as replacement for high surface area carbon for PEMFC catalysts. Electrochim. Acta 2014, 139, 21–28. [Google Scholar] [CrossRef]
- Mirshekari, G.; Rice, C. Effects of support particle size and Pt content on catalytic activity and durability of Pt/TiO2 catalyst for oxygen reduction reaction in proton exchange membrane fuel cells environment. J. Power Sources 2018, 396, 606–614. [Google Scholar] [CrossRef]
- Majlan, E.; Rohendi, D.; Daud, W.; Husaini, T.; Haque, M. Electrode for proton exchange membrane fuel cells: A review. Renew. Sustain. Energy Rev. 2018, 89, 117–134. [Google Scholar] [CrossRef]
- Chen, B.; Wang, J.; Yang, T.; Cai, Y.; Zhang, C.; Chan, S.H.; Yu, Y.; Tu, Z. Carbon corrosion and performance degradation mechanism in a proton exchange membrane fuel cell with dead-ended anode and cathode. Energy 2016, 106, 54–62. [Google Scholar] [CrossRef]
- Bing, Y.; Neburchilov, V.; Song, C.; Baker, R.; Guest, A.; Ghosh, D.; Ye, S.; Campbell, S.; Zhang, J. Effects of synthesis condition on formation of desired crystal structures of doped-TiO2/carbon composite supports for ORR electrocatalysts. Electrochim. Acta 2012, 77, 225–231. [Google Scholar] [CrossRef]
- Lee, S.W.; Choi, S.R.; Jang, J.; Park, G.-G.; Yu, S.H.; Park, J.-Y. Tolerance to carbon corrosion of various carbon structures as catalyst supports for polymer electrolyte membrane fuel cells. J. Mater. Chem. A 2019, 7, 25056–25065. [Google Scholar] [CrossRef]
- Takei, C.; Kakinuma, K.; Kawashima, K.; Tashiro, K.; Watanabe, M.; Uchida, M. Load cycle durability of a graphitized carbon black-supported platinum catalyst in polymer electrolyte fuel cell cathodes. J. Power Sources 2016, 324, 729–737. [Google Scholar] [CrossRef] [Green Version]
- Du, L.; Shao, Y.; Sun, J.; Yin, G.; Liu, J.; Wang, Y. Advanced catalyst supports for PEM fuel cell cathodes. Nano Energy 2016, 29, 314–322. [Google Scholar] [CrossRef] [Green Version]
- Ishitobi, H.; Kawatsu, Y.; Kudo, Y.; Nakagawa, N. Maximized specific activity for the methanol electrooxidation by the optimized PtRu-TiO2-carbon nano-composite structure. Int. J. Hydrogen Energy 2019, 44, 30743–30753. [Google Scholar] [CrossRef]
- O’Shea, V.A.; Galván, M.C.; Prats, A.E.; Campos-Martin, J.M.; Fierro, J.L. Direct evidence of the SMSI decoration effect: The case of Co/TiO2 catalyst. Chem. Commun. 2011, 47, 7131–7133. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, W.T.; della Mea, G.B.; Segala, M.; Baptista, D.L.; Escudero, C.; Pérez-Dieste, V.; Bernardi, F. Understanding the Strong Metal–Support Interaction (SMSI) Effect in CuxNi1−x/CeO2 (0 < x < 1) Nanoparticles for Enhanced Catalysis. ACS Appl. Nano Mater. 2019, 2, 2559–2573. [Google Scholar]
- Hsieh, B.-J.; Tsai, M.-C.; Pan, C.-J.; Su, W.-N.; Rick, J.; Chou, H.-L.; Lee, J.-F.; Hwang, B.-J. Tuning metal support interactions enhances the activity and durability of TiO2-supported Pt nanocatalysts. Electrochim. Acta 2017, 224, 452–459. [Google Scholar] [CrossRef]
- Wang, J.; Xu, M.; Zhao, J.; Fang, H.; Huang, Q.; Xiao, W.; Li, T.; Wang, D. Anchoring ultrafine Pt electrocatalysts on TiO2-C via photochemical strategy to enhance the stability and efficiency for oxygen reduction reaction. Appl. Catal. B Environ. 2018, 237, 228–236. [Google Scholar] [CrossRef]
- Tauster, S.; Fung, S.; Garten, R.L. Strong metal-support interactions. Group 8 noble metals supported on titanium dioxide. J. Am. Chem. Soc. 1978, 100, 170–175. [Google Scholar] [CrossRef]
- Li, Y.; Fan, Y.; Yang, H.; Xu, B.; Feng, L.; Yang, M.; Chen, Y. Strong metal-support interaction and catalytic properties of anatase and rutile supported palladium catalyst Pd/TiO2. Chem. Phys. Lett. 2003, 372, 160–165. [Google Scholar] [CrossRef]
- Montero-Ocampo, C.; Garcia, J.V.; Estrada, E.A. Comparison of TiO2 and TiO2-CNT as cathode catalyst supports for ORR. Int. J. Electrochem. Sci. 2013, 8, 12780–12800. [Google Scholar]
- Sá, J.; Bernardi, J.; Anderson, J.A. Imaging of low temperature induced SMSI on Pd/TiO2 catalysts. Catal. Lett. 2007, 114, 91–95. [Google Scholar] [CrossRef]
- Kouketsu, Y.; Mizukami, T.; Mori, H.; Endo, S.; Aoya, M.; Hara, H.; Nakamura, D.; Wallis, S. A new approach to develop the Raman carbonaceous material geothermometer for low-grade metamorphism using peak width. Isl. Arc 2014, 23, 33–50. [Google Scholar] [CrossRef]
- Claramunt, S.; Varea, A.; López-Díaz, D.; Velázquez, M.M.; Cornet, A.; Cirera, A. The importance of inter bands on the interpretation of the Raman spectrum of graphene oxide. J. Phys. Chem. C 2015, 119, 10123–10129. [Google Scholar] [CrossRef]
- López-Díaz, D.; Holgado, M.L.; García-Fierro, J.L.; Velázquez, M.M. Evolution of the Raman spectrum with the chemical composition of graphene oxide. J. Phys. Chem. C 2017, 121, 20489–20497. [Google Scholar] [CrossRef]
- Ali, S.; Li, Z.; Ali, W.; Zhang, Z.; Wei, M.; Qu, Y.; Jing, L. Synthesis of Au-decorated three-phase-mixed TiO2/phosphate modified active carbon nanocomposites as easily-recycled efficient photocatalysts for degrading high-concentration 2,4-DCP. RSC Adv. 2019, 9, 38414–38421. [Google Scholar] [CrossRef] [Green Version]
- Sasirekha, N.; Rajesh, B.; Chen, Y.-W. Synthesis of TiO2 sol in a neutral solution using TiCl4 as a precursor and H2O2 as an oxidizing agent. Thin Solid Films 2009, 518, 43–48. [Google Scholar] [CrossRef]
- Dambournet, D.; Belharouak, I.; Amine, K. Tailored preparation methods of TiO2 anatase, rutile, brookite: Mechanism of formation and electrochemical properties. Chem. Mater. 2010, 22, 1173–1179. [Google Scholar] [CrossRef]
- Xu, Z.; Zhuang, C.; Zou, Z.; Wang, J.; Xu, X.; Peng, T. Enhanced photocatalytic activity by the construction of a TiO2/carbon nitride nanosheets heterostructure with high surface area via direct interfacial assembly. Nano Res. 2017, 10, 2193–2209. [Google Scholar] [CrossRef]
- Vovk, E.I.; Kalinkin, A.V.; Smirnov, M.Y.; Klembovskii, I.O.; Bukhtiyarov, V.I. XPS study of stability and reactivity of oxidized Pt nanoparticles supported on TiO2. J. Phys. Chem. C 2017, 121, 17297–17304. [Google Scholar] [CrossRef]
- Pei, D.-N.; Gong, L.; Zhang, A.-Y.; Zhang, X.; Chen, J.-J.; Mu, Y.; Yu, H.-Q. Defective titanium dioxide single crystals exposed by high-energy {001} facets for efficient oxygen reduction. Nat. Commun. 2015, 6, 8696. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Yin, G.; Shao, Y.; Zhang, S.; Wang, Z.; Gao, Y. Effect of carbon black support corrosion on the durability of Pt/C catalyst. J. Power Sources 2007, 171, 331–339. [Google Scholar] [CrossRef]
- Odetola, C.; Trevani, L.; Easton, E.B. Enhanced activity and stability of Pt/TiO2/carbon fuel cell electrocatalyst prepared using a glucose modifier. J. Power Sources 2015, 294, 254–263. [Google Scholar] [CrossRef]
- Islam, J.; Kim, S.-K.; Kim, K.-H.; Lee, E.; Park, G.-G. Enhanced durability of Pt/C catalyst by coating carbon black with silica for oxygen reduction reaction. Int. J. Hydrogen Energy 2021, 46, 1133–1143. [Google Scholar] [CrossRef]
- Hussain, S.; Erikson, H.; Kongi, N.; Sarapuu, A.; Solla-Gullón, J.; Maia, G.; Kannan, A.M.; Alonso-Vante, N.; Tammeveski, K. Oxygen reduction reaction on nanostructured Pt-based electrocatalysts: A review. Int. J. Hydrogen Energy 2020, 45, 31775–31797. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jang, S.-J.; Kang, Y.C.; Hyun, J.-S.; Shin, T.H.; Lee, Y.W.; Roh, K.C. Hybrid Structure of TiO2-Graphitic Carbon as a Support of Pt Nanoparticles for Catalyzing Oxygen Reduction Reaction. Catalysts 2021, 11, 1196. https://doi.org/10.3390/catal11101196
Jang S-J, Kang YC, Hyun J-S, Shin TH, Lee YW, Roh KC. Hybrid Structure of TiO2-Graphitic Carbon as a Support of Pt Nanoparticles for Catalyzing Oxygen Reduction Reaction. Catalysts. 2021; 11(10):1196. https://doi.org/10.3390/catal11101196
Chicago/Turabian StyleJang, Su-Jin, Yun Chan Kang, Jin-Su Hyun, Tae Ho Shin, Young Wook Lee, and Kwang Chul Roh. 2021. "Hybrid Structure of TiO2-Graphitic Carbon as a Support of Pt Nanoparticles for Catalyzing Oxygen Reduction Reaction" Catalysts 11, no. 10: 1196. https://doi.org/10.3390/catal11101196