Promoting Photoelectrochemical Water Oxidation on Ti-Doped Fe2O3 Nanowires Photoanode by O2 Plasma Treatment
Abstract
:1. Introduction
2. Results and Discussion
2.1. Effect of O2 Plasma on Morphology, Structure, and Optical Properties of the Electrodes
2.2. Effect of O2 Plasma on the PEC Performance of Ti-Fe2O3 Photoanodes
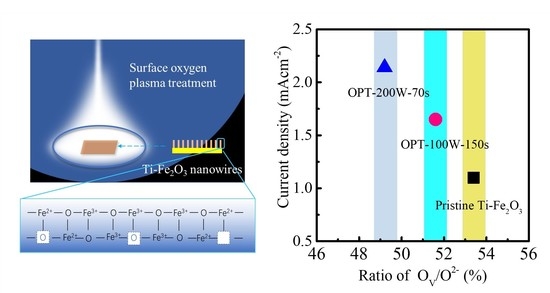
2.3. Mechanism of O2 Plasma Treatment on Promoting PEC Performance
3. Materials and Methods
3.1. Materials
3.2. Preparation of Ti-Doped Fe2O3 Photoanodes
3.2.1. Preparation of Ti-Fe2O3 Nanowires Array on FTO Substrate
3.2.2. O2 Plasma Treated Ti-Doped Hematite Nanowires
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.-H.; Kronawitter, C.X.; Wheeler, D.A.; Guo, P.; Lindley, S.A.; Jiang, J.; Zhang, J.-Z.; Guo, L.; Mao, S.-S. Physical and photoelectrochemical characterization of Ti-doped hematite photoanodes prepared by solution growth. J. Mater.Chem. A 2013, 1, 14498–14506. [Google Scholar] [CrossRef]
- Franking, R.; Li, L.; Lukowski, M.A.; Meng, F.; Tan, Y.; Hamers, R.J.; Jin, S. Facile post-growth doping of nanostructured hematite photoanodes for enhanced photoelectrochemical water oxidation. Energy Environ. Sci. 2013, 6, 500–512. [Google Scholar] [CrossRef]
- Mazzaro, R.; Bibi, S.B.; Natali, M.; Bergamini, G.; Morandi, V.; Ceroni, P.; Vomiero, A. Hematite nanostructures: An old material for a new story. Simultaneous photoelectrochemical oxidation of benzylamine and hydrogen production through Ti doping. Nano Energy 2019, 61, 36–46. [Google Scholar] [CrossRef]
- Feng, F.; Li, C.; Jian, J.; Li, F.; Xu, Y.-X.; Wang, H.-Q.; Jia, L.-C. Gradient Ti-doping in hematite photoanodes for enhanced photoelectrochemical performance. J. Power Sour. 2020, 449, 227473. [Google Scholar] [CrossRef]
- Upul Wijayantha, K.G.; Saremi-Yarahmadi, S.; Peter, L.M. Kinetics of oxygen evolution at α-Fe2O3 photoanodes: A study by photoelectrochemical impedance spectroscopy. Phys. Chem. Chem. Phys. 2011, 13, 5264–5270. [Google Scholar] [CrossRef] [Green Version]
- Pendlebury, S.R.; Barroso, M.; Cowan, A.J.; Sivula, K.; Tang, J.W.; Grätzel, M.; Klug, D.; Durrant, J.R. Dynamics of photogenerated holes in nanocrystalline α-Fe2O3 electrodes for water oxidation probed by transient absorption spectroscopy. Chem. Commun. 2011, 47, 716–718. [Google Scholar] [CrossRef]
- Klahr, B.; Gimenez, S.; Fabregat-Santiago, F.; Hamann, T.; Bisquert, J. Water oxidation at hematite photoelectrodes: The role of surface states. J. Am. Chem. Soc. 2012, 134, 4294–4302. [Google Scholar] [CrossRef] [Green Version]
- Hamann, T.W. Splitting water with rust: Hematite photoelectrochemistry. Dalton Trans. 2012, 41, 7830–7834. [Google Scholar] [CrossRef]
- McDonald, K.J.; Choi, K.-S. Synthesis and photoelectrochemical properties of Fe2O3/ZnFe2O4 composite photoanodes for use in solar water oxidation. Chem. Mater. 2011, 23, 4863–4869. [Google Scholar] [CrossRef]
- Steier, L.; Herraiz-Cardona, I.; Gimenez, S.; Fabregat-Santiago, F.; Bisquert, J.; Tilley, S.D.; Grätzel, M. Understanding the role of underlayers and overlayers in thin film hematite photoanodes. Adv. Funct. Mater. 2014, 24, 7681–7688. [Google Scholar] [CrossRef]
- Zhong, D.-K.; Sun, J.-W.; Inumaru, H.; Gamelin, D.R. Solar water oxidation by composite catalyst/α-Fe2O3 photoanodes. J. Am. Chem. Soc. 2009, 131, 6086–6087. [Google Scholar] [CrossRef] [PubMed]
- Zhong, D.-K.; Gamelin, D.R. Photoelectrochemical water oxidation by cobalt catalyst (“Co–Pi”)/α-Fe2O3 composite photoanodes: Oxygen evolution and resolution of a kinetic bottleneck. J. Am. Chem. Soc. 2010, 132, 4202–4207. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.-W.; Xu, Z.; Yan, S.-C.; Zou, Z.-G. Tuning the ion permeability of an Al2O3 coating layer on Fe2O3 photoanodes for improved photoelectrochemical water oxidation. J. Mater. Chem. A 2017, 5, 8402–8407. [Google Scholar] [CrossRef]
- Hu, Z.-F.; Shen, Z.-R.; Yu, J.C. Covalent fixation of surface oxygen atoms on hematite photoanode for enhanced water oxidation. Chem. Mater. 2016, 28, 564–572. [Google Scholar] [CrossRef]
- Zhu, C.-Q.; Li, C.-L.; Zheng, M.-J.; Delaunay, J.J. Plasma-induced oxygen vacancies in ultrathin hematite nanoflakes promoting photoelectrochemical water oxidation. ACS Appl. Mater. Interfaces 2015, 7, 22355–22363. [Google Scholar] [CrossRef]
- Li, M.-Y.; Zhang, Z.-Y.; Lyu, F.-Y.; He, X.-J.; Liang, Z.-H.; Balogun, M.S.; Lu, X.-H.; Fang, P.-P.; Tong, Y.-X. Facile hydrothermal synthesis of three dimensional hematite nanostructures with enhanced water splitting performance. Electrochim. Acta 2015, 186, 95–100. [Google Scholar] [CrossRef]
- Kyesmen, P.I.; Nombona, N.; Diale, M. Modified annealing approach for preparing multi-layered hematite thin films for photoelectrochemical water splitting. Mater. Res. Bull. 2020, 131, 110964. [Google Scholar] [CrossRef]
- Wu, F.-K.; Xie, J.-L.; You, Y.-E.; Zhao, Z.-Y.; Wang, L.-L.; Chen, X.-Y.; Yang, P.-P.; Huang, Y.-L. Cobalt metal–organic framework ultrathin cocatalyst overlayer for improved photoelectrochemical activity of Ti-doped hematite. ACS Appl. Energy Mater. 2020, 3, 4867–4876. [Google Scholar] [CrossRef]
- Mo, R.; Liu, Q.; Li, H.-X.; Yang, S.; Zhong, J.-X. Photoelectrochemical water oxidation in α-Fe2O3 thin films enhanced by a controllable wet-chemical Ti-doping strategy and Co–Pi co-catalyst modification. J. Mater. Sci. Mater. El. 2019, 30, 21444–21453. [Google Scholar] [CrossRef]
- Cao, D.-P.; Zhang, J.-B.; Wang, A.-C.; Yu, X.-H.; Mi, B.-X. Fabrication of Cr-doped SrTiO3/Ti-doped α-Fe2O3 photoanodes with enhanced photoelectrochemical properties. J. Mater. Sci. Technol. 2020, 56, 189–195. [Google Scholar] [CrossRef]
- Wang, G.-M.; Yang, Y.; Ling, Y.-C.; Wang, H.-Y.; Lu, X.-H.; Pu, Y.-C.; Zhang, J.-Z.; Tong, Y.-X.; Li, Y. An electrochemical method to enhance the performance of metal oxides for photoelectrochemical water oxidation. J. Mater. Chem. A 2016, 4, 2849–2855. [Google Scholar] [CrossRef]
- Dotan, H.; Sivula, K.; Grätzel, M.; Rothschild, A.; Warren, S.C. Probing the photoelectrochemical properties of hematite (α-Fe2O3) electrodes using hydrogen peroxide as a hole scavenger. Energy Environ. Sci. 2011, 4, 958–964. [Google Scholar] [CrossRef]
- Klotz, D.; Grave, D.A.; Rothschild, A. Accurate determination of the charge transfer efficiency of photoanodes for solar water splitting. Phys. Chem. Chem. Phys. 2017, 19, 20383–20392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bohn, C.D.; Agrawal, A.K.; Walter, E.C.; Vaudin, M.D.; Herzing, A.A.; Haney, P.M.; Talin, A.A.; Szalai, V.A. Effect of tin doping on α-Fe2O3 photoanodes for water splitting. J. Phys. Chem. C 2012, 116, 15290–15296. [Google Scholar] [CrossRef]
- Orazem, M.E.; Tribollet, B. Electrochemical Impedance Spectroscopy; Wiley: New York, NY, USA, 2001; pp. 225–230. [Google Scholar]
- Wilhelm, S.M.; Yun, K.S.; Ballenger, L.W.; Hackerman, N. Semiconductor properties of iron oxide electrodes. J. Electrochem. Soc. 1979, 126, 419–424. [Google Scholar] [CrossRef]
- Turner, J.E.; Hendewerk, M.; Parmeter, J.; Neiman, D.; Somorjai, G.A. The characterization of doped iron oxide electrodes for the photodissociation of water: Stability, optical, and electronic properties. J. Electrochem. Soc. 1984, 131, 1777–1783. [Google Scholar] [CrossRef]
- Gao, S.; Wang, D.; Wang, Y.-L.; Li, C.; Liu, Y.-C.; Suzuki, N.; Terashima, C.; Fujishima, A.; Zhang, X.-T. Activating titanium dopants in hematite photoanode by rapid thermal annealing for enhancing photoelectrochemical water oxidation. Electrochim. Acta 2019, 318, 746–753. [Google Scholar] [CrossRef]
- Fujii, T.; de Groot, F.M.F.; Sawatzky, G.A.; Voogt, F.C.; Hibma, T.; Okada, K. In situ XPS analysis of various iron oxide films grown by NO2-assisted molecular-beam epitaxy. Phys. Rev. B Condens. Matter Mater. Phys. 1999, 59, 3195–3202. [Google Scholar] [CrossRef] [Green Version]
- Lu, J.; Jiao, X.; Chen, D.; Li, W. Solvothermal synthesis and characterization of Fe3O4 and γ-Fe2O3 nanoplates. J. Phys. Chem. C 2009, 113, 4012–4017. [Google Scholar] [CrossRef]
- Chae, S.Y.; Rahman, G.; Joo, O.S. Elucidation of the structural and charge separation properties of titanium-doped hematite films deposited by electrospray method for photoelectrochemical water oxidation. Electrochim. Acta 2019, 297, 784–793. [Google Scholar] [CrossRef]
- Kawabe, T.; Tabata, K.; Suzuki, E.; Yamaguchi, Y.; Nagasawa, Y. Electronic states of chemisorbed oxygen species and their mutually related studies on SnO2 thin film. J. Phys. Chem. B 2001, 105, 4239–4244. [Google Scholar] [CrossRef]
- Bao, J.; Zhang, X.; Fan, B.; Zhang, J.; Zhou, M.; Yang, W.; Hu, X.; Wang, H.; Pan, B.; Xie, Y. Ultrathin spinel-structured nanosheets rich in oxygen deficiencies for enhanced electrocatalytic water oxidation. Angew. Chem. Int. Ed. 2015, 54, 7399–7404. [Google Scholar] [CrossRef] [PubMed]
- Moir, J.; Soheilnia, N.; Liao, K.; O’Brien, P.; Tian, Y.; Burch, K.S.; Ozin, G.A. Activation of ultrathin films of hematite for photoelectrochemical water splitting via H2 treatment. ChemSusChem 2015, 8, 1557–1567. [Google Scholar] [CrossRef]
- Janowitz, C.; Scherer, V.; Mohamed, M.; Krapf, A.; Dwelk, H.; Manzke, R.; Galazka, Z.; Uecker, R.; Irmacher, K.; Fornari, R.; et al. Experimental electronic structure of In2O3 and Ga2O3. New J. Phys. 2011, 13, 085014. [Google Scholar] [CrossRef]
- Lee, J.; Han, S. Thermodynamics of native point defects in α-Fe2O3: An Ab initio study. Phys. Chem. Chem. Phys. 2013, 15, 18906–18914. [Google Scholar] [CrossRef]
- Rioult, M.; Stanescu, D.; Fonda, E.; Barbier, A.; Magnan, H. Oxygen vacancies engineering of iron oxides films for solar water splitting. J. Phys. Chem. C 2016, 120, 7482–7490. [Google Scholar] [CrossRef]
- Fu, Z.; Jiang, T.; Zhang, L.; Liu, B.; Wang, D.; Wang, L.; Xie, T. Surface treatment with Al3+ on a Ti-doped α-Fe2O3 nanorod array photoanode for efficient photoelectrochemical water splitting. J. Mater. Chem. A 2014, 2, 13705–13712. [Google Scholar] [CrossRef]
- Vayssieres, L.; Beermann, N.; Lindquist, S.E.; Hagfeldt, A. Controlled aqueous chemical growth of oriented three-dimensional crystalline nanorod arrays: Application to iron(III) oxides. Chem. Mater. 2001, 13, 233–235. [Google Scholar] [CrossRef]
- Li, C.; Wang, D.; Suzuki, N.; Terashima, C.; Liu, Y.-C.; Fujishima, A.; Zhang, X.-T. A coral-like hematite photoanode on a macroporous SnO2:Sb substrate for enhanced photoelectrochemical water oxidation. Electrochim. Acta 2020, 360, 137012. [Google Scholar] [CrossRef]







| Circuit Element | Rs (Ω) | Rct (Ω) | Cbulk (F) | Csc (F) |
|---|---|---|---|---|
| Pristine Ti-Fe2O3 | 9.87 | 143.49 | 8.77 × 10−5 | 3.98 × 10−4 |
| OPT−100W−150s | 9.66 | 114.25 | 9.46 × 10−5 | 7.99 × 10−4 |
| OPT−200W−70s | 9.41 | 103.67 | 9.48 × 10−5 | 7.14 × 10−4 |
| Sample | Binding Energy (eV) | ||||
|---|---|---|---|---|---|
| Fe 2p1/2 | Fe 2p3/2 | Ti 2p1/2 | Ti 2p3/2 | O1s (Main Peak) | |
| Pristine Ti-Fe2O3 | 724.78 | 711.3 | 464.23 | 458.39 | 530.22 |
| OPT-100W-150s | 724.78 | 711.3 | 464.53 | 458.64 | 530.31 |
| OPT-200W-70s | 725 | 711.5 | 464.53 | 458.75 | 530.45 |
| Sample | Ratio of Ov/O2− |
|---|---|
| Pristine Ti-Fe2O3 | 53.4% |
| OPT-100W-150s | 51.6% |
| OPT-200W-70s | 49.8% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.; Wang, D.; Gu, J.; Liu, Y.; Zhang, X. Promoting Photoelectrochemical Water Oxidation on Ti-Doped Fe2O3 Nanowires Photoanode by O2 Plasma Treatment. Catalysts 2021, 11, 82. https://doi.org/10.3390/catal11010082
Li C, Wang D, Gu J, Liu Y, Zhang X. Promoting Photoelectrochemical Water Oxidation on Ti-Doped Fe2O3 Nanowires Photoanode by O2 Plasma Treatment. Catalysts. 2021; 11(1):82. https://doi.org/10.3390/catal11010082
Chicago/Turabian StyleLi, Chuang, Dan Wang, Jiangli Gu, Yichun Liu, and Xintong Zhang. 2021. "Promoting Photoelectrochemical Water Oxidation on Ti-Doped Fe2O3 Nanowires Photoanode by O2 Plasma Treatment" Catalysts 11, no. 1: 82. https://doi.org/10.3390/catal11010082