Experimental and Kinetic Study on the Production of Furfural and HMF from Glucose
Abstract
:1. Introduction
2. Results
2.1. Synergistic Effect of Lewis and Brønsted Acids
2.2. Kinetics Model
2.3. Effect of Solvent
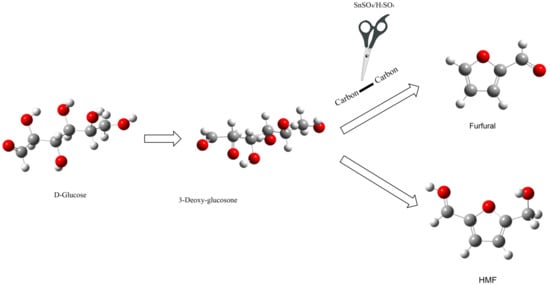
2.4. Possible Reaction Pathway
3. Materials and Methods
3.1. Materials
3.2. Catalytic Reactions
3.3. Determination of Glucose, Furfural, HMF, and Formaldehyde
3.4. Kinetic Calculation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Nomenclature
| A | pre-exponential factor (min−1) |
| k | reaction rate constant (min−1) |
| E | activation energy (kJ/mol) |
| R | (kJ mol−1 K−1) |
| T | temperature (K) |
| kG | glucose degradation rate constant (min−1) |
| k1 | furfural formation rate constant (min−1) |
| k2 | HMF formation rate constant (min−1) |
| k3 | degradation rate constant for furfural (min−1) |
| k4 | degradation rate constant for HMF (min−1) |
| CGLU | initial concentration of glucose (mol L−1) |
| CHMF | initial concentration of HMF (mol L−1) |
| CFUR | initial concentration of furfural (mol L−1) |
| Ea1 | activation energy for furfural production (kJ mol−1) |
| Ea2 | activation energy for HMF production (kJ mol−1) |
| Ea3 | activation energy for furfural degradation (kJ mol−1) |
| Ea4 | activation energy for HMF degradation (kJ mol−1) |
| Ea5 | activation energy for glucose degradation (kJ mol−1) |
References
- Lakshmanan, V.I.; Roy, R.; Gorain, B. Renewable energy. In Innovations and Breakthroughs in the Gold and Silver Industries: Concepts, Applications and Future Trends; Springer: Berlin, Germany, 2019; ISBN 9783030325497. [Google Scholar]
- Świątek, K.; Gaag, S.; Klier, A.; Kruse, A.; Sauer, J.; Steinbach, D. Acid hydrolysis of lignocellulosic biomass: Sugars and furfurals formation. Catalysts 2020, 10, 437. [Google Scholar] [CrossRef] [Green Version]
- Ng, F.T.T.; Dalai, A.K. Catalysis for the production of sustainable fuels and chemicals. Catalysts 2020, 10, 388. [Google Scholar] [CrossRef] [Green Version]
- Esteban, J.; Yustos, P.; Ladero, M. Catalytic processes from biomass-derived hexoses and pentoses: A recent literature overview. Catalysts 2018, 8, 637. [Google Scholar] [CrossRef] [Green Version]
- Hao, W.; Zhao, G.; Tang, X.; Liu, S.; Lin, L.; Sun, Y.; Hu, L. Catalytic conversion of biomass-derived carbohydrates into fuels and chemicals via furanic aldehydes. RSC Adv. 2012, 2, 11184. [Google Scholar] [CrossRef]
- Mariscal, R.; Maireles-Torres, P.; Ojeda, M.; Sádaba, I.; López Granados, M. Furfural: A renewable and versatile platform molecule for the synthesis of chemicals and fuels. Energy Environ. Sci. 2016, 9, 1144–1189. [Google Scholar] [CrossRef]
- Kohli, K.; Prajapati, R.; Sharma, B.K. Bio-based chemicals from renewable biomass for integrated biorefineries. Energies 2019, 12, 233. [Google Scholar] [CrossRef] [Green Version]
- Buntara, T.; Noel, S.; Phua, P.H.; Melián-Cabrera, I.; De Vries, J.G.; Heeres, H.J. Caprolactam from renewable resources: Catalytic conversion of 5-hydroxymethylfurfural into caprolactone. Angew. Chem. Int. Ed. 2011, 50, 7083–7087. [Google Scholar] [CrossRef]
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Zheng, C. Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]
- Lee, D.; Owens, V.N.; Boe, A.; Jeranyama, P. Composition of herbaceous biomass feedstocks. Cellulose 2007, 24, 16. [Google Scholar]
- Dedes, G.; Karnaouri, A.; Topakas, E. Novel routes in transformation of lignocellulosic biomass to furan platform chemicals: From pretreatment to enzyme catalysis. Catalysts 2020, 10, 743. [Google Scholar] [CrossRef]
- Chatterjee, C.; Pong, F.; Sen, A. Chemical conversion pathways for carbohydrates. Green Chem. 2015, 17, 40–71. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, X.; Zheng, H.; Li, X.; Zhu, Y.; Li, Y. Mechanistic insights on catalytic conversion fructose to furfural on beta zeolite via selective carbon-carbon bond cleavage. Mol. Catal. 2019, 463, 130–139. [Google Scholar] [CrossRef]
- Dutta, S.; De, S.; Saha, B. Advances in biomass transformation to 5-hydroxymethylfurfural and mechanistic aspects. Biomass Bioenergy 2013, 55, 355–369. [Google Scholar] [CrossRef]
- Nikolla, E.; Román-Leshkov, Y.; Moliner, M.; Davis, M.E. “One-pot” synthesis of 5-(hydroxymethyl)furfural from carbohydrates using tin-beta zeolite. ACS Catal. 2011, 1, 408–410. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Xi, G.; Chen, Z.; Jiang, D.; Yu, H.; Wang, X. Highly selective conversion of glucose into furfural over modified zeolites. Chem. Eng. J. 2017, 307, 868–876. [Google Scholar] [CrossRef]
- Wang, K.; Ye, J.; Zhou, M.; Liu, P.; Liang, X.; Xu, J.; Jiang, J. Selective conversion of cellulose to levulinic acid and furfural in sulfolane/water solvent. Cellulose 2017. [Google Scholar] [CrossRef]
- Yang, F.; Fu, J.; Mo, J.; Lu, X. Synergy of Lewis and Brønsted acids on catalytic hydrothermal decomposition of hexose to levulinic acid. Energy Fuels 2013, 27, 6973–6978. [Google Scholar] [CrossRef]
- Gürbüz, E.I.; Gallo, J.M.R.; Alonso, D.M.; Wettstein, S.G.; Lim, W.Y.; Dumesic, J.A. Conversion of hemicellulose into furfural using solid acid catalysts in γ-valerolactone. Angew. Chem. Int. Ed. 2013, 52, 1270–1274. [Google Scholar] [CrossRef]
- Jin, F.; Enomoto, H. Rapid and highly selective conversion of biomass into value-added products in hydrothermal conditions: Chemistry of acid/base-catalysed and oxidation reactions. Energy Environ. Sci. 2011, 4, 382–397. [Google Scholar] [CrossRef]
- Wrigstedt, P.; Keskiväli, J.; Leskelä, M.; Repo, T. The role of salts and Brønsted acids in lewis acid-catalyzed aqueous-phase glucose dehydration to 5-hydroxymethylfurfural. ChemCatChem 2015, 7, 501–507. [Google Scholar] [CrossRef]
- Mellmer, M.A.; Sener, C.; Gallo, J.M.R.; Luterbacher, J.S.; Alonso, D.M.; Dumesic, J.A. Solvent effects in acid-catalyzed biomass conversion reactions. Angew. Chem. Int. Ed. 2014, 53, 11872–11875. [Google Scholar] [CrossRef] [PubMed]
- Walker, T.W.; Chew, A.K.; Li, H.; Demir, B.; Zhang, Z.C.; Huber, G.W.; Van Lehn, R.C.; Dumesic, J.A. Universal kinetic solvent effects in acid-catalyzed reactions of biomass-derived oxygenates. Energy Environ. Sci. 2018, 11, 617–628. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Wei, M.; Wang, F.; Wei, L.; Yin, X.; Jiang, J.; Wang, K. Effective and facile conversion of bamboo into platform chemicals over SnCl4 in a sulfolane/water solution. J. Energy Inst. 2020. [Google Scholar] [CrossRef]
- Hu, B.; Lu, Q.; Jiang, X.; Dong, X.; Cui, M.; Dong, C.; Yang, Y. Pyrolysis mechanism of glucose and mannose: The formation of 5-hydroxymethyl furfural and furfural. J. Energy Chem. 2018, 27, 486–501. [Google Scholar] [CrossRef] [Green Version]
- Asakawa, M.; Shrotri, A.; Kobayashi, H.; Fukuoka, A. Solvent basicity controlled deformylation for the formation of furfural from glucose and fructose. Green Chem. 2019, 21, 6146–6153. [Google Scholar] [CrossRef]
- Cui, J.; Tan, J.; Deng, T.; Cui, X.; Zhu, Y.; Li, Y. Conversion of carbohydrates to furfural via selective cleavage of the carbon–carbon bond: The cooperative effects of zeolite and solvent. Green Chem. 2016, 18, 1619–1624. [Google Scholar] [CrossRef]
- Wang, T.; Nolte, M.W.; Shanks, B.H. Catalytic dehydration of C6 carbohydrates for the production of hydroxymethylfurfural (HMF) as a versatile platform chemical. Green Chem. 2014, 16, 548–572. [Google Scholar] [CrossRef]
- Wang, C.; Yang, G.; Zhang, X.; Shao, L.; Lyu, G.; Mao, J.; Liu, S.; Xu, F. A kinetic study on the hydrolysis of corncob residues to levulinic acid in the FeCl3–NaCl system. Cellulose 2019. [Google Scholar] [CrossRef]
- Weiqi, W.; Shubin, W. Experimental and kinetic study of glucose conversion to levulinic acid catalyzed by synergy of Lewis and Brønsted acids. Chem. Eng. J. 2017, 307, 389–398. [Google Scholar] [CrossRef]
- Atanda, L.; Konarova, M.; Ma, Q.; Mukundan, S.; Shrotri, A.; Beltramini, J. High yield conversion of cellulosic biomass into 5-hydroxymethylfurfural and a study of the reaction kinetics of cellulose to HMF conversion in a biphasic system. Catal. Sci. Technol. 2016, 6, 6257–6266. [Google Scholar] [CrossRef]





| Entry | Brønsted Acid (mol/L) | Lewis Acid (mol/L) | Furfural Production (%) | HMF Production (%) | Glucose Conversion (%) | Furfural Selectivity (%) | HMF Selectivity (%) |
|---|---|---|---|---|---|---|---|
| 1 | n.a | n.a | n.a | n.a | n.a | n.a | n.a |
| 2 | H2SO4 (0.009) | n.a | 7 ± 0.8 | 8 ± 1.4 | 54 ± 3.9 | 13 ± 0.6 | 15 ± 1.5 |
| 3 | H2SO4 (0.018) | n.a | 12 ± 1.4 | 6 ± 0.5 | 49 ± 4.3 | 24 ± 0.7 | 12 ± 0.1 |
| 4 | H2SO4 (0.018) | MnSO4 (0.014) | 4 ± 0.6 | 15 ± 1.2 | 58 ± 7.2 | 14 ± 0.2 | 26 ± 1.2 |
| 5 | H2SO4 (0.018) | CuSO4 (0.014) | 24 ± 0.1 | 32 ± 0.3 | 92 ± 2.4 | 26 ± 0.6 | 35 ± 0.6 |
| 6 | H2SO4 (0.018) | SnSO4 (0.014) | 33 ± 2.5 | 21 ± 1.8 | 99 ± 0.2 | 33 ± 2.5 | 22 ± 1.3 |
| 7 | H2SO4 (0.018) | SnSO4 (0.028) | 29 ± 1.4 | 12 ± 0.3 | 84 ± 2.9 | 36 ± 0.5 | 14 ± 0.1 |
| 8 | HCl (0.018) | SnSO4 (0.014) | 15 ± 1.7 | 37 ± 2.3 | 79 ± 4.2 | 19 ± 1.1 | 47 ± 0.4 |
| 9 | H3PO4 (0.018) | SnSO4 (0.014) | 1 ± 0.5 | 1 ± 0.6 | 10 ± 0.5 | 10 ± 4.5 | 10 ± 5.5 |
| 10 | HNO3 (0.018) | SnSO4 (0.014) | 20 ± 2.1 | 36 ± 3.0 | 76 ± 1.9 | 27 ± 3.2 | 48 ± 5.0 |
| 10 | n.a | SnSO4 (0.014) | n.a | n.a | Trace | n.a | n.a |
| 11 | n.a | SnSO4 (0.028) | n.a | n.a | Trace | n.a | n.a |
| Temp. | 433 K | 443 K | 453 K | 463 K |
|---|---|---|---|---|
| kG | 0.103 | 0.169 | 0.254 | 0.338 |
| k1 | 0.0518 | 0.0849 | 0.0976 | 0.121 |
| k2 | 0.0466 | 0.0713 | 0.076 | 0.124 |
| k3 | 0.0185 | 0.0194 | 0.0205 | 0.0213 |
| k4 | 0.0329 | 0.0761 | 0.115 | 0.138 |
| k5 | 0.0046 | 0.0128 | 0.0804 | 0.093 |
| k1/k2 | 1.1 | 1.2 | 1.3 | 1.0 |
| k5/kG | 0.04 | 0.08 | 0.32 | 0.30 |
| k1/k3 | 2.8 | 4.4 | 4.8 | 5.7 |
| k2/k4 | 1.4 | 0.9 | 0.7 | 0.9 |
| k4/k3 | 1.8 | 3.9 | 5.6 | 6.5 |
| Temperature | kG | k1 | k2 | k3 | k4 | k5 |
|---|---|---|---|---|---|---|
| Ea (kJ mol−1) | 66.9 | 44.9 | 49.9 | 8.0 | 79.0 | 181.5 |
| A (min−1) | 1.17 × 107 | 1.49 × 104 | 5.02 × 104 | 5.91 × 100 | 1.32 × 108 | 3.92 × 1019 |
| R2 | 0.991 | 0.935 | 0.932 | 0.997 | 0.921 | 0.927 |
| GVL/Water Ratio | 90% | 85% | 80% |
|---|---|---|---|
| kG | 0.315 | 0.197 | 0.169 |
| k1 | 0.0578 | 0.07679 | 0.0849 |
| k2 | 0.0486 | 0.0695 | 0.0713 |
| k3 | 0.0006 | 0.00484 | 0.0194 |
| k4 | 0.0748 | 0.08966 | 0.0761 |
| k5 | 0.2186 | 0.0507 | 0.0128 |
| k1/k2 | 1.2 | 1.1 | 1.2 |
| k5/kG | 0.69 | 0.26 | 0.08 |
| k1/k3 | 96.3 | 15.9 | 4.4 |
| k2/k4 | 0.6 | 0.8 | 0.9 |
| k4/k3 | 124.7 | 18.5 | 3.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, O.; Zhang, Y.; Wang, P.; Liu, L.; Wang, Q.; Yang, N.; Li, W.; Champagne, P.; Yu, H. Experimental and Kinetic Study on the Production of Furfural and HMF from Glucose. Catalysts 2021, 11, 11. https://doi.org/10.3390/catal11010011
He O, Zhang Y, Wang P, Liu L, Wang Q, Yang N, Li W, Champagne P, Yu H. Experimental and Kinetic Study on the Production of Furfural and HMF from Glucose. Catalysts. 2021; 11(1):11. https://doi.org/10.3390/catal11010011
Chicago/Turabian StyleHe, Ouwen, Yangfan Zhang, Pan Wang, Lina Liu, Qian Wang, Nan Yang, Wenjie Li, Pascale Champagne, and Hongbing Yu. 2021. "Experimental and Kinetic Study on the Production of Furfural and HMF from Glucose" Catalysts 11, no. 1: 11. https://doi.org/10.3390/catal11010011