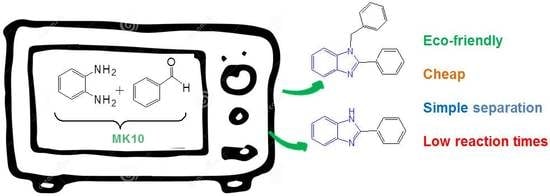
Montmorillonite K10: An Efficient Organo-Heterogeneous Catalyst for Synthesis of Benzimidazole Derivatives
Abstract
:1. Introduction
2. Results
3. Materials and Methods
3.1. General Methods
3.2. General Procedure for the Synthesis of 1,2-Substituted Benzimidazoles 1a–8a
3.3. General Procedure for the Synthesis of 2-Substituted Benzimidazoles 1b–8b
3.4. Catalyst Recycling
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Emerson, G.; Brink, N.G.; Holly, F.W.; Koniuszy, F.; Heyl, D.; Folker, K. Vitamin B12. VIII. Vitamin B12 -Like Activity of 5,6-Dimethylbenzimidazole and Tests on related compounds. J. Am. Chem. Soc. 1950, 72, 3084–3085. [Google Scholar] [CrossRef]
- Kubo, K.; Oda, K.; Kaneko, T.; Satoh, H.; Nohara, A. Synthesis of 2-(4- Fluoroalkoxy-2-pyridyl) methyl] sulfinyl]-1H-benzimidazoles as Antiulcer Agents. Chem. Pharm. Bull. 1990, 38, 2853–2858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uchida, M.; Chihiro, M.; Morita, S.; Yamashita, H.; Yamasaki, K.; Kanbe, T.; Yabuuchi, Y.; Nakagawz, K. Synthesis and Antiulcer Activity of 4- Substituted 8-[(2-Benzimidazolyl) sulfinylmethyl]-1, 2, 3, 4-tetrahydroquinolines and Related Compounds. Chem. Pharm. Bull. 1990, 38, 1575–1586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grassi, A.; Ippen, J.; Bruno, M.; Thomas, G.; Bay, P. A thiazolylamino benzimidazole derivative with gastroprotective properties in the rat. Eur. J. Pharmacol. 1991, 195, 251–259. [Google Scholar] [CrossRef]
- Ozkay, Y.; Tunali, Y.; Karaca, H.; Isikdag, I. Antimicrobial activity and a SAR study of some novel benzimidazole derivatives bearing hydrazones moiety. Eur. J. Med. Chem. 2010, 45, 3293–3298. [Google Scholar] [CrossRef]
- Algul, O.; Karabulut, A.; Canacankatan, N.; Gorur, A.; Sucu, N.; Vezir, O. Apoptotic and anti-angiogenic effects of benzimidazole compounds: Relationship with oxidative stress mediated ischemia/reperfusion injury in rat hind limb. Antiinflamm Antiallergy Agents Med. Chem. 2012, 11, 267–275. [Google Scholar] [CrossRef]
- Thakuria, H.; Das, G. An expeditious one-pot solvent-free synthesis of benzimidazole derivatives. ARKIVOC 2008, 15, 321–328. [Google Scholar]
- Rithe, S.R.; Jagtap, R.S.; Ubarhande, S.S. One Pot Synthesis of Substituted Benzimidazole Derivatives And Their Characterization. RASAYAN J. Chem. 2015, 8, 213–217. [Google Scholar]
- Liyan, F.; Wen, C.; Lulu, K. Highly chemoselective synthesis of benzimidazoles in Sc(OTf)3-catalyzed system. Heterocycles 2015, 91, 2306–2314. [Google Scholar]
- Bahrami, K.; Khodaei, M.M.; Kavianinia, I. H2O2/HCl as a new and efficient system for synthesis of 2-substituted benzimidazoles. J. Chem. Res. 2006, 12, 783–784. [Google Scholar] [CrossRef]
- Ma, H.; Han, X.; Wang, Y.; Wang, J. A simple and efficient method for synthesis of benzimidazoles using FeBr3 or Fe(NO3)3·9H2O as catalyst. ChemInform. 2007, 38, 1821–1825. [Google Scholar] [CrossRef]
- Du, L.-H.; Wang, Y.-G. A rapid and efficient synthesis of benzimidazoles using hypervalent iodine as oxidant. Synthesis 2007, 5, 675–678. [Google Scholar]
- Sontakke, V.A.; Ghosh, S.; Lawande, P.P.; Chopade, B.A.; Shinde, V.S. A simple, efficient synthesis of 2-aryl benzimidazoles using silica supported periodic acid catalyst and evaluation of anticancer activity. ISRN Org. Chem. 2013, 2013, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, K.R.; Satyanarayana, P.V.V.; Reddy, B.S. NaHSO4-SiO2 promoted synthesis of benzimidazole derivatives. Arch. Appl. Sci. Res. 2012, 4, 1517–1521. [Google Scholar]
- Venkateswarlu, Y.; Kumar, S.R.; Leelavathi, P. Facile and efficient one-pot synthesis of benzimidazoles using lanthanum chloride. Org. Med. Chem. Lett. 2013, 3, 2–8. [Google Scholar]
- Martins, G.M.; Puccinelli, T.; Gariani, R.A.; Xavier, F.R.; Silveira, C.C.; Mendes, S.R. Facile and efficient aerobic one-pot synthesis of benzimidazoles using Ce(NO3)3·6H2O as promoter. Tetrahedron Lett. 2017, 58, 1969–1972. [Google Scholar] [CrossRef]
- Mobinikhaledi, A.; Hamta, A.; Kalhor, M.; Shariatzadeh, M. Simple Synthesis and Biological Evaluation of Some Benzimidazoles Using Sodium Hexafluroaluminate, Na3 AlF6, as an Efficient Catalyst. Iran. J. Pharm. Res. 2014, 13, 95–101. [Google Scholar]
- Birajdar, S.S.; Hatnapure, G.D.; Keche, A.P.; Kamble, V.M. Synthesis of 2-substituted-1 H-benzo[d]imidazoles through oxidative cyclization of O-phenylenediamine and substituted aldehydes using dioxanedibromide. Res. J. Pharm. Biol. Chem. Sci. 2014, 5, 487–493. [Google Scholar]
- Srinivasulu, R.; Kumar, K.R.; Satyanarayana, P.V.V. Facile and Efficient Method for Synthesis of Benzimidazole Derivatives Catalyzed by Zinc Triflate. Green Sustain. Chem. 2014, 4, 33–37. [Google Scholar] [CrossRef] [Green Version]
- Sehyun, P.; Jaehun, J.; Eun, J.C. Visible-Light-Promoted Synthesis of Benzimidazoles. J. Org. Chem. 2014, 352, 4148–4154. [Google Scholar]
- Vishvanath, D.P.; Ketan, P.P. Synthesis of Benzimidazole and Benzoxazole Derivatives Catalyzed by Nickel Acetate as Organometallic Catalyst. Int. J. ChemTech Res. 2014, 8, 457–465. [Google Scholar]
- Procopio, A.; De Nino, A.; Nardi, M.; Oliverio, M.; Paonessa, R.; Pasceri, R. A New Microwave-Assisted Organocatalytic Solvent-Free Synthesis of Optically Enriched Michael Adducts. Synlett 2010, 12, 1849–1853. [Google Scholar] [CrossRef]
- Deng, Q.; Wang, R. Heterogeneous MOF catalysts for the synthesis of trans-4,5-diaminocyclopent-2-enones from furfural and secondary amines. Catal. Commun. 2019, 120, 11–16. [Google Scholar]
- Thomas, J.M.; Raja, R.; Lewis, D.W. Single-site heterogeneous catalysts. Angew. Chem. Int. Ed. 2005, 44, 6456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Osman, A.I.; Abu-Dahrieh, J.K.; McLaren, M.; Laffir, F.; Rooney, D.W. Characterisation of Robust Combustion Catalyst from Aluminium Foil Waste. ChemistrySelect 2018, 3, 1545–1550. [Google Scholar] [CrossRef] [Green Version]
- Goswami, M.; Dutta, M.M.; Phukan, P. Sulfonic-acid-functionalized activated carbon made from tea leaves as green catalyst for synthesis of 2-substituted benzimidazole and benzothiazole. Res. Chem. Intermed. 2018, 44, 1597–1615. [Google Scholar] [CrossRef]
- Nelso, W.M. Green Solvents for Chemistry Perspectives and Practice; Oxford University Press: Oxford, UK, 2004. [Google Scholar]
- Mikami, K. Green Reaction Media in Organic Synthesis; Blackwell: Tokyo, Japan, 2005. [Google Scholar]
- Clark, J.H.; Tavener, S.J. Alternative Solvents: Shades of Green. Org. Process Res. Dev. 2007, 11, 149–155. [Google Scholar] [CrossRef]
- Ballini, R.; Bosica, G.; Carloni, L.; Maggi, R.; Sartori, G. Zeolite HSZ-360 as a new reusable catalyst for the direct acetylation of alcohols and phenols under solventless conditions. Tetrahedron Lett. 1998, 39, 6049–6052. [Google Scholar] [CrossRef]
- Bartoli, G.; Dalpozzo, R.; De Nino, A.; Maiuolo, L.; Nardi, M.; Procopio, A.; Tagarelli, A. Cerium(III) Triflate versus Cerium(III) Chloride: Anion Dependence of Lewis Acid Behavior in the Deprotection of PMB Ethers. Eur. J. Org. Chem. 2004, 10, 2176–2180. [Google Scholar] [CrossRef]
- Procopio, A.; Cravotto, G.; Oliverio, M.; Costanzo, P.; Nardi, M.; Paonessa, R. An Eco-Sustainable Erbium(III)-Catalysed Method for Formation/Cleavage of O-tert-butoxy carbonates. Green Chem. 2011, 13, 436–443. [Google Scholar] [CrossRef]
- Oliverio, M.; Costanzo, P.; Macario, A.; De Luca, G.; Nardi, M.; Procopio, A. A Bifuctional Heterogeneous Catalyst Erbium-Based: A Cooperative Route Towards C-C Bond Formation. Molecules 2014, 19, 10218–10229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Procopio, A.; Das, G.; Nardi, M.; Oliverio, M.; Pasqua, L. A Mesoporous Er(III)-MCM-41 Catalyst for the Cyanosilylation of Aldehydes and Ketones under Solvent-free Conditions. ChemSusChem 2008, 1, 916–919. [Google Scholar] [CrossRef] [PubMed]
- Procopio, A.; Costanzo, P.; Curini, M.; Nardi, M.; Oliverio, M.; Sindona, G. Erbium(III) Chloride in Ethyl Lactate as a Smart Ecofriendly System for Efficient and Rapid Stereoselective Synthesis of trans-4,5-Diaminocyclopent-2-enones. ACS Sustain Chem Eng. 2013, 1, 541–544. [Google Scholar] [CrossRef]
- Virot, M.; Tomao, V.; Ginies, C.; Chemat, F. Total lipid extraction of food using d-limonene as an alternative to n-hexane. Chromatographia 2008, 68, 311–313. [Google Scholar] [CrossRef]
- Lapkin, A.; Plucinski, P.K.; Cutler, M. Comparative assessment of technologies for extraction of artemisinin. J. Nat. Prod. 2006, 69, 1653–1664. [Google Scholar] [CrossRef]
- Pereira, C.S.M.; Silva, V.M.T.M.; Rodrigues, A.E. Ethyl lactate as a solvent: Properties, applications and production processes. Green Chem. 2011, 13, 2658–2671. [Google Scholar] [CrossRef]
- García, J.I.; García-Marín, H.; Pires, E. Glycerol based solvents: Synthesis, properties and applications. Green Chem. 2014, 16, 1007–1033. [Google Scholar] [CrossRef] [Green Version]
- Nardi, M.; Oliverio, M.; Costanzo, P.; Sindona, G.; Procopio, A. Eco-friendly stereoselective reduction of α,β-unsaturated carbonyl compounds by Er(OTf)3/NaBH4 in 2-MeTHF. Tetrahedron 2015, 71, 1132–1135. [Google Scholar] [CrossRef]
- Nardi, M.; Herrera Cano, N.; De Nino, A.; Di Gioia, M.L.; Maiuolo, L.; Oliverio, M.; Santiago, A.; Sorrentino, D.; Procopio, A. An eco-friendly tandem tosylation/Ferrier N-glycosylation of amines catalyzed by Er(OTf)3 in 2-MeTHF. Tetrahedron Lett. 2017, 58, 1721–1726. [Google Scholar] [CrossRef]
- Weishi Miao, W.; Chan, T.H. Ionic-Liquid-Supported Synthesis: A Novel Liquid-Phase Strategy for Organic Synthesis. Acc. Chem. Res. 2006, 39, 897–908. [Google Scholar]
- Abbott, A.P.; Davies, D.L.; Capper, G.; Rasheed, R.K.; Tambyrajah, V. Ionic Liquids and Their Use As solvents. U.S. Patent 7,183,433, 27 February 2007. [Google Scholar]
- Di Gioia, M.L.; Costanzo, P.; De Nino, A.; Maiuolo, L.; Nardi, M.; Olivito, F.; Procopio, A. Simple and efficient Fmoc removal in ionic liquid. RSC Adv. 2017, 7, 36482–36491. [Google Scholar] [CrossRef] [Green Version]
- De Nino, A.; Maiuolo, L.; Merino, P.; Nardi, M.; Procopio, A.; Roca-Lõpez, D.; Russo, B.; Algieri, V. Efficient organocatalyst supported on a simple ionic liquid as a recoverable system for the asymmetric diels-alder reaction in the presence of water. ChemCatChem 2015, 7, 830–835. [Google Scholar] [CrossRef]
- Abbott, A.P.; Capper, G.; Davies, D.L.; Rasheed, R.K.; Tambyrajah, V. Novel solvent properties of choline chloride/urea mixtures. Chem. Commun. 2003, 1, 70–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gorke, J.T.; Srienc, F.; Kazlauskas, R.J. Hydrolase-catalyzed biotransformations in deep eutectic solvents. Chem. Commun. 2008, 10, 1235–1237. [Google Scholar] [CrossRef]
- Smith, E.L.; Abbott, A.P.; Ryder, K.S. Deep Eutectic Solvents (DESs) and their applications. Chem. Rev. 2014, 114, 11060–11082. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paiva, A.; Craveiro, R.; Aroso, I.; Martins, M.; Reis, R.L.; Duarte, A.R.C. Natural deep eutectic solvents—Solvents for the 21st century. ACS Sustain. Chem. Eng. 2014, 2, 1063–1071. [Google Scholar] [CrossRef]
- Di Gioia, M.L.; Cassano, R.; Costanzo, P.; Herrera Cano, N.; Maiuolo, L.; Nardi, M.; Nicoletta, F.P.; Oliverio, M.; Procopio, A. Green Synthesis of Privileged Benzimidazole Scaffolds Using Active Deep Eutectic Solvent. Molecules 2019, 24, 2885. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonacci, S.; Di Gioia, M.L.; Costanzo, P.; Maiuolo, L.; Tallarico, S.; Nardi, M. Natural Deep Eutectic Solvent as Extraction Media for the Main Phenolic Compounds from Olive Oil Processing Wastes. Antioxidants 2020, 9, 513. [Google Scholar] [CrossRef]
- Leitner, W.; Poliakoff, M. Supercritical fluids in green chemistry. Green Chem. 2008, 10, 730. [Google Scholar]
- Carlès, P. A brief review of the thermophysical properties of supercritical fluids. J. Supercrit. Fluids 2010, 53, 2–11. [Google Scholar] [CrossRef]
- Lindström, U.M. Stereoselective Organic Reactions in Water. Chem. Rev. 2002, 10, 2751–2772. [Google Scholar] [CrossRef] [PubMed]
- Procopio, A.; Gaspari, M.; Nardi, M.; Oliverio, M.; Tagarelli, A.; Sindona, G. Simple and efficient MW-assisted cleavage of acetals and ketals in pure water. Tetrahedron Lett. 2007, 48, 8623–8627. [Google Scholar] [CrossRef]
- Procopio, A.; Gaspari, M.; Nardi, M.; Oliverio, M.; Rosati, O. Highly efficient and versatile chemoselective addition of amines to epoxides in water catalyzed by erbium(III) triflate. Tetrahedron Lett. 2008, 49, 2289–2293. [Google Scholar] [CrossRef]
- Simon, M.O.; Li, C.J. Green chemistry oriented organic synthesis in water. Chem. Soc. Rev. 2012, 41, 1415–1427. [Google Scholar] [CrossRef] [PubMed]
- Oliverio, M.; Costanzo, P.; Paonessa, R.; Nardi, M.; Procopio, A. Catalyst-free tosylation of lipophilic alcohols in water. RSC Adv. 2013, 3, 2548–2552. [Google Scholar] [CrossRef]
- Nardi, M.; Herrera Cano, N.; Costanzo, P.; Oliverio, M.; Sindona, G.; Procopio, A. Aqueous MW eco-friendly protocol for amino group protection. RSC Adv. 2015, 5, 18751–18760. [Google Scholar] [CrossRef]
- Nardi, M.; Di Gioia, M.L.; Costanzo, P.; De Nino, A.; Maiuolo, L.; Oliverio, M.; Olivito, F.; Procopio, A. Selective acetylation of small biomolecules and their derivatives catalyzed by Er(OTf)3. Catalysts 2017, 7, 269. [Google Scholar] [CrossRef] [Green Version]
- Nardi, M.; Costanzo, P.; De Nino, A.; Di Gioia, M.L.; Olivito, F.; Sindona, G.; Procopio, A. Water excellent solvent for the synthesis of bifunctionalized cyclopentenones from furfural. Green Chem. 2017, 19, 5403–5411. [Google Scholar] [CrossRef]
- Olivito, F.; Costanzo, P.; Di Gioia, M.L.; Nardi, M.; Oliverio, M.; Procopio, A. Efficient synthesis of organic thioacetate in water. Org. Biomol. Chem. 2018, 16, 7753–7759. [Google Scholar] [CrossRef]
- Procopio, A.; De Luca, G.; Nardi, M.; Oliverio, M.; Paonessa, R. General MW-assisted grafting of MCM-41: Study of the dependence on time dielectric heating and solvent. Green Chem. 2009, 11, 770–773. [Google Scholar] [CrossRef]
- Estevão, M.S.; Afonso, C.A.M. Synthesis of trans-4,5-diaminocyclopent-2-enones from furfural catalyzed by Er(III) immobilized on silica. Tetrahedron Lett. 2017, 58, 302–304. [Google Scholar] [CrossRef]
- Senthilkumar, S.; Maru, M.S.; Somani, R.S.; Bajaj, H.C.; Neogi, S. Unprecedented NH2-MIL-101(Al)/n-Bu4NBr system as solvent-free heterogeneous catalyst for efficient synthesis of cyclic carbonates via CO2 cycloaddition. Dalton Trans. 2018, 47, 418–428. [Google Scholar] [CrossRef] [PubMed]
- Mason, T.J. Sonochemistry: Current uses and future prospects in the chemical and processing industries. Philos. Trans. R. Soc. Lond. A 1999, 357, 355–369. [Google Scholar] [CrossRef]
- Cravotto, G.; Cintas, P. The combined use of microwaves and ultrasound: Improved tools in process chemistry and organic synthesis. Chem. Eur. J. 2007, 13, 1902–1909. [Google Scholar] [CrossRef] [PubMed]
- Je˘selnik, M.; Varma, R.S.; Polanca, S.; Kocevar, M. Catalyst-free reactions under solvent-free conditions: Microwave-assisted synthesis of heterocyclic hydrazones below the melting points of neat reactants. Chem. Commun. 2001, 18, 1716–1717. [Google Scholar] [CrossRef]
- Kappe, O. Controlled microwave heating in modern organic synthesis. Angew. Chem. Int. Ed. 2004, 43, 6250–6284. [Google Scholar] [CrossRef]
- Desai, K.R. Green Chemistry Microwave Synthesis, 1st ed.; Himalaya Publication House: New Delhi, India, 2005; p. 20. [Google Scholar]
- Horikoshi, S.; Serpone, N. Role of microwaves in heterogeneous catalytic systems. Catal. Sci. Technol. 2014, 4, 1197–1210. [Google Scholar]
- Procopio, A.; Dalpozzo, R.; De Nino, A.; Maiuolo, L.; Nardi, M.; Romeo, G. Mild and efficient method for the cleavage of benzylidene acetals by using erbium (III) triflate. Org. Biomol. Chem. 2005, 3, 4129–4133. [Google Scholar] [CrossRef]
- Oliverio, M.; Costanzo, P.; Nardi, M.; Calandruccio, C.; Salerno, R.; Procopio, A. Tunable microwave-assisted method for the solvent-free and catalyst-free peracetylation of natural products. Beilstein J. Org. Chem. 2016, 12, 2222–2233. [Google Scholar] [CrossRef] [Green Version]
- Maiuolo, L.; Merino, P.; Algieri, V.; Nardi, M.; Di Gioia, M.L.; Russo, B.; Delso, I.; Tallarida, M.A.; De Nino, A. Nitrones and nucleobase-containing spiro-isoxazolidines derived from isatin and indanone: Solvent-free microwave-assisted stereoselective synthesis and theoretical calculations. RSC Adv. 2017, 7, 48980–48988. [Google Scholar] [CrossRef] [Green Version]
- Bortolini, O.; D’Agostino, M.; De Nino, A.; Maiuolo, L.; Nardi, M.; Sindona, G. Solvent-free, microwave assisted 1,3-cycloaddition of nitrones with vinyl nucleobases for the synthesis of N,O-nucleosides. Tetrahedron 2008, 64, 8078–8081. [Google Scholar] [CrossRef]
- Procopio, A.; Gaspari, M.; Nardi, M.; Oliverio, M.; Romeo, R. MW-assisted Er(OTf)3 -catalyzed mild cleavage of isopropylidene acetals in Tricky substrates. Tetrahedron Lett. 2008, 49, 1961–1964. [Google Scholar] [CrossRef]
- Nardi, M.; Bonacci, S.; De Luca, G.; Maiuolo, J.; Oliverio, M.; Sindona, G.; Procopio, A. Biomimetic synthesis and antioxidant evaluation of 3,4-DHPEA-EDA [2-(3,4-hydroxyphenyl) ethyl (3S,4E)-4-formyl-3-(2-oxoethyl)hex-4-enoate]. Food Chem. 2014, 162, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Oliverio, M.; Nardi, M.; Cariati, L.; Vitale, E.; Bonacci, S.; Procopio, A. “on Water” MW-Assisted Synthesis of Hydroxytyrosol Fatty Esters. ACS Sustain. Chem. Eng. 2016, 4, 661–665. [Google Scholar] [CrossRef]
- Maiuolo, L.; De Nino, A.; Algieri, V.; Nardi, M. Microwave-assisted 1,3-dipolar cyclo-addition: Recent advances in synthesis of isoxazolidines. Mini-Rev. Org. Chem. 2017, 14, 136–142. [Google Scholar] [CrossRef] [Green Version]
- Nardi, M.; Bonacci, S.; Cariati, L.; Costanzo, P.; Oliverio, M.; Sindona, G.; Procopio, A. Synthesis and antioxidant evaluation of lipophilic oleuropein aglycone derivatives. Food Funct. 2017, 8, 4684–4692. [Google Scholar] [CrossRef]
- Costanzo, P.; Calandruccio, C.; Di Gioia, M.L.; Nardi, M.; Oliverio, M.; Procopio, A. First multicomponent reaction exploiting glycerol carbonate synthesis. J. Clean. Prod. 2018, 202, 504–509. [Google Scholar] [CrossRef]
- Costanzo, P.; Bonacci, S.; Cariati, L.; Nardi, M.; Oliverio, M.; Procopio, A. Simple and efficient sustainable semi-synthesis of oleacein [2-(3,4-hydroxyphenyl) ethyl (3S,4E)-4-formyl-3-(2-oxoethyl)hex-4-enoate] as potential additive for edible oils. Food Chem. 2018, 245, 410–414. [Google Scholar] [CrossRef]
- Paonessa, R.; Nardi, M.; Di Gioia, M.L.; Olivito, F.; Oliverio, M.; Procopio, A. Eco-friendly synthesis of lipophilic EGCG derivatives and antitumor and antioxidant evaluation. Nat. Prod. Commun. 2018, 9, 1117–1122. [Google Scholar] [CrossRef] [Green Version]
- Li, J.-T.; Xing, C.-Y.; Li, T.-S. An efficient and environmentally friendly method for synthesis of arylmethylenemalononitrile catalyzed by Montmorillonite K10–ZnCl2 under ultrasound irradiation. J. Chem. Technol. Biotechnol. 2004, 79, 1275–1278. [Google Scholar] [CrossRef]
- Bhattacharyya, K.G.; Gupta, S.S. Adsorption of a few heavy metals on natural and modified kaolinite and montmorillonite: A review. Adv. Colloid Interface Sci. 2008, 140, 114–131. [Google Scholar] [CrossRef] [PubMed]
- Kaur, N.; Kishore, D. Montmorillonite: An efficient, heterogeneous and green catalyst for organic synthesis. J. Chem. Pharm. Res. 2012, 4, 991–1015. [Google Scholar]
- Kumar, B.S.; Dhakshinamoorthy, A.; Pitchumani, K. K10 montmorillonite clays as environmentally benign catalysts for organic reactions. Catal. Sci. Technol. 2014, 4, 2378–2396. [Google Scholar] [CrossRef]
- Hechelski, M.; Ghinet, A.; Brice Louvel, B.; Dufrenoy, P.; Rigo, B.; Daïch, A.; Waterlot, C. From Conventional Lewis Acids to Heterogeneous Montmorillonite K10: Eco-Friendly Plant-Based Catalysts Used as Green Lewis Acids. ChemSusChem 2018, 11, 1249–1277. [Google Scholar] [CrossRef] [PubMed]
- Rostamizadeh, S.; Amani, A.M.; Aryan, R.; Ghaieni, H.R.; Norouzi, L. Very fast and efficient synthesis of some novel substituted 2-arylbenzimidazoles in water using ZrOCl2·nH2O on montmorillonite K10 as catalyst. Mon. Chem. 2009, 140, 547–552. [Google Scholar] [CrossRef]
- Hashemi, M.M.; Eftekhari-Sis, B.; Abdollahifar, A.; Khalili, B. ZrOCl2·8H2O on montmorillonite K10 accelerated conjugate addition of amines to α,β-unsaturated alkenes under solvent-free conditions. Tetrahedron 2006, 62, 672–677. [Google Scholar] [CrossRef]
- Borah, S.J.; Das, D.K. Modified Montmorillonite: An Active Heterogeneous Catalyst for the Synthesis of Benzimidazoles. J. Chem. Pharm. Res. 2018, 10, 118–123. [Google Scholar]
- Bonacci, S.; Nardi, M.; Costanzo, P.; De Nino, A.; Di Gioia, M.L.; Oliverio, M.; Procopio, A. Montmorillonite K10-Catalyzed Solvent-Free Conversion of Furfural into Cyclopentenones. Catalysts 2019, 9, 301. [Google Scholar] [CrossRef] [Green Version]
- Hegedüs, A.; Hell, Z.; Potor, A. Zeolite-Catalyzed Environmentally Friendly Synthesis of Benzimidazole Derivatives. Synth. Commun. 2009, 36, 3625–3630. [Google Scholar] [CrossRef]
- Khanday, W.A.; Tomar, R. Conversion of zeolite—A in to various ion-exchanged catalytic forms and their catalytic efficiency for the synthesis of benzimidazole. Catal. Commun. 2014, 43, 141–145. [Google Scholar] [CrossRef]
- Saberi, A. Efficient synthesis of Benzimidazoles using zeolite, alumina and silica gel under microwave irradiation. Iran. J. Sci. Technol. 2015, 39, 7–10. [Google Scholar]
- Nardi, M.; Cozza, A.; Maiuolo, L.; Oliverio, M.; Procopio, A. 1,5-Benzoheteroazepines through eco-friendly general condensation reactions. Tetrahedron Lett. 2011, 52, 4827–4834. [Google Scholar] [CrossRef]
- Nardi, M.; Cozza, A.; De Nino, A.; Oliverio, M.; Procopio, A. One-pot synthesis of dibenzo[b,e][1,4]diazepin-1-ones. Synthesis 2012, 44, 800–804. [Google Scholar] [CrossRef]
- Oliverio, M.; Costanzo, P.; Nardi, M.; Rivalta, I.; Procopio, A. Facile ecofriendly synthesis of monastrol and its structural isomers via biginelli reaction. ACS Sustain. Chem. Eng. 2014, 2, 1228–1233. [Google Scholar] [CrossRef]
- Herrera Cano, N.; Uranga, J.G.; Nardi, M.; Procopio, A.; Wunderlin, D.A.; Santiago, A.N. Selective and eco-friendly procedures for the synthesis of benzimidazole derivatives. The role of the Er(OTf)3 catalyst in the reaction selectivity. Beilstein J. Org. Chem. 2016, 12, 2410–2419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Nino, A.; Maiuolo, L.; Nardi, M.; Pasceri, R.; Procopio, A.; Russo, B. Development of one-pot three component reaction for the synthesis of N′-aryl-N-cyanoformamidines, essential precursors of formamidine pesticides family. Arab. J. Chem. 2016, 9, 32–37. [Google Scholar] [CrossRef] [Green Version]


| Entry | MK10 wt (%) b | Molar Ratio o-PDA: Benzaldehyde | Temp (°C) | Time (min) | Conversion (%)c | Selectivity (%) d |
|---|---|---|---|---|---|---|
| 1 | 10 | 1:1 | rt | 120 | 19.3 | 12.0 |
| 2 | 10 | 1:2 | rt | 120 | 20.9 | 53.0 |
| 3 | 10 | 1:2 | 60 | 120 | 79.6 | 65.1 |
| 4 | 10 | 1:1 | 80 | 120 | 80.9 | 33.3 |
| 5 | 10 | 1:1 | 100 | 60 | 99.9 | 38.3 |
| 6 | 10 | 1:2 | 100 | 60 | 99.9 | 75.0 |
| 7 | - | 1:2 | 100 | 90 | 45.0 | 49.0 |
| 8 e | 20 | 1:1 | 60 | 5 | 99.9 | 18.2 |
| 9 e | 20 | 1:2 | 60 | 5 | 99.9 | 98.5 |
| Entry | Aldehyde | Product | Conversion (%) | Yield (%) b |
|---|---|---|---|---|
| 1 |  |  1a | 99.9 | 95.0 |
| 2 |  |  2a | 98.7 | 96.6 |
| 3 |  |  3a | 99.9 | 99.6 |
| 4 c |  |  4a | 982 | 0 |
| 5 c |  |  5a | 97.3 | 0 |
| 6 |  |  6a | 91.0 | 90.8 |
| 7 |  |  7a | 97.8 | 95.1 |
| 8 |  |  8a | 96.8 | 93.8 |
| Entry | Aldehyde | Product | Conversion (%) | Yield (%) b | |
|---|---|---|---|---|---|
| 1 |  |  1b | 99.9 | 95.0 | |
| 2 |  |  2b | 95.9 | 97.8 | |
| 3 |  |  3b | 99.9 | 99.0 | |
| 4 |  |  4b | 90.6 | 98.3 | |
| 5 |  |  5b | 89.6 | 97.3 | |
| 6 |  |  6b | 91.0 | 90.8 | |
| 7 |  |  7b | 97.8 | 94.8 | |
| 8 |  |  8b | 96.8 | 94.1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonacci, S.; Iriti, G.; Mancuso, S.; Novelli, P.; Paonessa, R.; Tallarico, S.; Nardi, M. Montmorillonite K10: An Efficient Organo-Heterogeneous Catalyst for Synthesis of Benzimidazole Derivatives. Catalysts 2020, 10, 845. https://doi.org/10.3390/catal10080845
Bonacci S, Iriti G, Mancuso S, Novelli P, Paonessa R, Tallarico S, Nardi M. Montmorillonite K10: An Efficient Organo-Heterogeneous Catalyst for Synthesis of Benzimidazole Derivatives. Catalysts. 2020; 10(8):845. https://doi.org/10.3390/catal10080845
Chicago/Turabian StyleBonacci, Sonia, Giuseppe Iriti, Stefano Mancuso, Paolo Novelli, Rosina Paonessa, Sofia Tallarico, and Monica Nardi. 2020. "Montmorillonite K10: An Efficient Organo-Heterogeneous Catalyst for Synthesis of Benzimidazole Derivatives" Catalysts 10, no. 8: 845. https://doi.org/10.3390/catal10080845