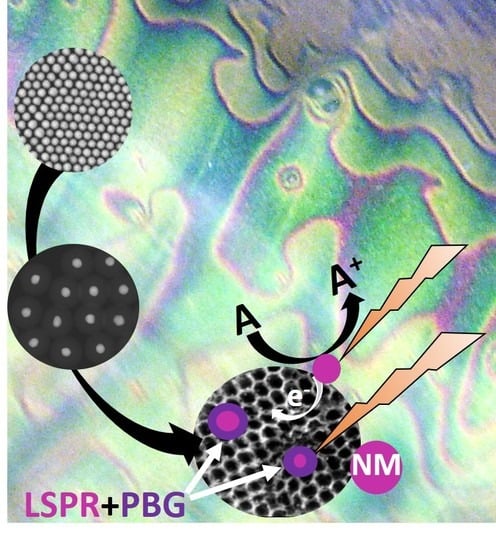
Photonic Crystals for Plasmonic Photocatalysis
Abstract
:1. Introduction
2. Plasmonic Photocatalysis
3. Photonic Crystals (PCs)
4. NM-Based PCs
4.1. Preparation of PCs-Based Plasmonic Photocatalysts
4.2. Photocatalytic Applications
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| 1D | one-dimensional |
| 3D | three-dimensional |
| AVD | atomic vapor deposition |
| BM | biomorphic |
| CB | conduction band |
| CVD | chemical vapor deposition |
| DAP | decahedral anatase particles |
| ETs | electron traps |
| FTO | fluorine-doped tin oxide |
| HNSs | hollow nanospheres |
| IO | inverse opal |
| IPCE | incident photon-to-electron conversion efficiency |
| LSPR | localized surface plasmon resonance |
| MB | methylene blue |
| NIR | near infrared |
| NM | noble metals |
| NPs | nanoparticles |
| NRs | nanorods |
| OAP | octahedral anatase particles |
| PCs | photonic crystals |
| PBG | photonic bandgap |
| PRET | plasmon resonance energy transfer |
| PMMA | polymethyl methacrylate |
| PS | polystyrene |
| QDs | quantum dots |
| r-GO | reduced graphene oxide |
| TNTs | titania nanotubes |
| UV | ultraviolet |
| VB | valence band |
| Vis | visible light |
References
- Hoffmann, M.R.; Martin, S.T.; Choi, W.Y.; Bahnemann, D.W. Environmental applications of semiconductor photocatalysis. Chem. Rev. 1995, 95, 69–96. [Google Scholar] [CrossRef]
- Nakata, K.; Fujishima, A. TiO2 photocatalysis: Design and applications. J. Photochem. Photobiol. 2012, 13, 169–189. [Google Scholar] [CrossRef]
- Ohtani, B. Photocatalysis A to Z—What we know and what we do not know in a scientific sense. J. Photochem. Photobiol. 2010, 11, 157–178. [Google Scholar] [CrossRef] [Green Version]
- Abe, R. Recent progress on photocatalytic and photoelectrochemical water splitting under visible light irradiation. J. Photochem. Photobiol. 2010, 11, 179–209. [Google Scholar] [CrossRef]
- Wang, K.L.; Janczarek, M.; Wei, Z.S.; Raja-Mogan, T.; Endo-Kimura, M.; Khedr, T.M.; Ohtani, B.; Kowalska, E. Morphology- and crystalline composition-governed activity of titania-based photocatalysts: Overview and perspective. Catalysts 2019, 9, 1054. [Google Scholar] [CrossRef] [Green Version]
- Herrmann, J.M. Heterogeneous photocatalysis: Fundamentals and applications to the removal of various types of aqueous pollutants. Catal. Today 1999, 53, 115–129. [Google Scholar] [CrossRef]
- Zaleska, A. Doped-TiO2: A review. Rec. Patent. Eng. 2008, 2, 157–164. [Google Scholar] [CrossRef]
- Kraeutler, B.; Bard, A.J. Heterogeneous photocatalytic preparation of supported catalysts. Photodeposition of platinum on TiO2 powder and other substrates. J. Am. Chem. Soc. 1978, 100, 4317–4318. [Google Scholar] [CrossRef]
- Nishimoto, S.I.; Ohtani, B.; Kagiya, T. Photocatalytic dehydrogenation of aliphatic-alcohols by aqueous suspensions of platinized titanium-dioxide. J. Chem. Soc. Farad. Trans. 1985, 81, 2467–2474. [Google Scholar] [CrossRef]
- Pichat, P.; Herrmann, J.M.; Disdier, J.; Courbon, H.; Mozzanega, M.N. Photocatalytic hydrogen production from aliphatic alcohols over a bifunctional platinum on titanium dioxide catalyst. Nouv. J. Chim. 1981, 5, 627–636. [Google Scholar]
- Bahnemann, D.W.; Mönig, J.; Chapman, R. Efficient photocatalysis of the irreversible one-electron and two-electron reduction of halothane on platinized colloidal titanium dioxide in aqueous suspension. J. Phys. Chem. 1987, 91, 3782–3788. [Google Scholar] [CrossRef]
- Wang, K.L.; Wei, Z.S.; Ohtani, B.; Kowalska, E. Interparticle electron transfer in methanol dehydrogenation on platinum-loaded titania particles prepared from P25. Catal. Today 2018, 303, 327–333. [Google Scholar] [CrossRef]
- Macyk, W.; Burgeth, G.; Kisch, H. Photoelectrochemical properties of platinum (IV) chloride surface modified TiO2. Photochem. Photobiol. Sci. 2003, 2, 322–328. [Google Scholar] [CrossRef]
- Kowalska, E.; Remita, H.; Colbeau-Justin, C.; Hupka, J.; Belloni, J. Modification of titanium dioxide with platinum ions and clusters: Application in photocatalysis. J. Phys. Chem. 2008, 112, 1124–1131. [Google Scholar] [CrossRef]
- Tian, Y.; Tatsuma, T. Mechanisms and applications of plasmon-induced charge separation at TiO2 films loaded with gold nanoparticles. J. Am. Chem. Soc. 2005, 127, 7632–7637. [Google Scholar] [CrossRef] [PubMed]
- Verbruggen, S.W. TiO2 photocatalysis for the degradation of pollutants in gas phase: From morphological design to plasmonic enhancement. J. Photochem. Photobiol. 2015, 24, 64–82. [Google Scholar] [CrossRef]
- Kowalska, E. Plasmonic photocatalysis. In Gold Nanoparticles for Physics, Chemistry and Biology, 2nd ed.; Louis, C., Pluchery, O., Eds.; World Scientific: Singapore, 2017; pp. 319–364. [Google Scholar]
- Ueno, K.; Misawa, H. Surface plasmon-enhanced photochemical reactions. J. Photochem. Photobiol. 2013, 15, 31–52. [Google Scholar] [CrossRef]
- Sarina, S.; Waclawik, E.R.; Zhu, H.Y. Photocatalysis on supported gold and silver nanoparticles under ultraviolet and visible light irradiation. Green Chem. 2013, 15, 1814–1833. [Google Scholar] [CrossRef]
- Kowalska, E. Plasmonic Photocatalysts. Available online: https://www.mdpi.com/journal/catalysts/special_issues/plasmonic_photocatal (accessed on 2 July 2020).
- Verbruggen, S.W. Functional Plasmonic Nanostructures. Available online: https://www.mdpi.com/journal/nanomaterials/special_issues/functional_plasmonic (accessed on 2 July 2020).
- Kominami, H.; Tanaka, A.; Hashimoto, K. Mineralization of organic acids in aqueous suspension of gold nanoparticles supported on cerium (IV) oxide powder under visible light irradiation. Chem. Commun. 2010, 46, 1287–1289. [Google Scholar] [CrossRef]
- Thimsen, E.; Le Formal, F.; Gratzel, M.; Warren, S.C. Influence of plasmonic Au nanoparticles on the photoactivity of Fe2O3 electrodes for water splitting. Nano Lett. 2011, 11, 35–43. [Google Scholar] [CrossRef]
- Yu, H.; Ming, H.; Zhang, H.; Li, H.; Pan, K.; Liu, Y.; Wang, F.; Gong, J.; Kang, Z. Au/ZnO nanocomposite: Facile fabrication and enhanced photocatalytic activity for degradation of benzene. Mater. Chem. Phys. 2012, 137, 113–117. [Google Scholar] [CrossRef]
- Lan, J.Y.; Zhou, X.M.; Liu, G.; Yu, J.G.; Zhang, J.C.; Zhi, L.J.; Nie, G.J. Enhancing photocatalytic activity of one-dimensional KNbO3 nanowires by Au nanoparticles under ultraviolet and visible-light. Nanoscale 2011, 3, 5161–5167. [Google Scholar] [CrossRef]
- Kashyap, T.; Biswasi, S.; Pal, A.R.; Choudhury, B. Unraveling the catalytic and plasmonic roles of g-C3N4 supported Ag and Au nanoparticles under selective photoexcitation. ACS Sustain. Chem. Eng. 2019, 7, 19295–19302. [Google Scholar] [CrossRef]
- Xu, H.; Li, H.M.; Xia, J.X.; Yin, S.; Luo, Z.J.; Liu, L.; Xu, L. One-pot synthesis of visible-light-driven plasmonic photocatalyst Ag/AgCl in ionic liquid. ACS Appl. Mater. Interfaces 2011, 3, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.L.; Zhang, J.F.; Lv, J.L.; Dai, K.; Liang, C.H. Plasmonic Ag2MoO4/AgBr/Ag composite: Excellent photocatalytic performance and possible photocatalytic mechanism. Appl. Surf. Sci. 2017, 396, 791–798. [Google Scholar] [CrossRef]
- Del Tedesco, A.; Piotto, V.; Sponchia, G.; Hossain, K.; Litti, L.; Peddis, D.; Scarso, A.; Meneghetti, M.; Benedetti, A.; Riello, P. Zirconia-based magnetoplasmonic nanocomposites: A new nanotool for magnetic-guided separations with SERS identification. ACS Appl. Nano Mater. 2020, 3, 1232–1241. [Google Scholar] [CrossRef]
- Subramanian, V.; Wolf, E.; Kamat, P.V. Semiconductor-metal composite nanostructures. To what extent do metal nanoparticles improve the photocatalytic activity of TiO2 films? J. Phys. Chem. 2001, 105, 11439–11446. [Google Scholar] [CrossRef]
- Rodriguez-Gonzalez, V.; Zanella, R.; del Angel, G.; Gomez, R. MTBE visible-light photocatalytci decomposition over Au/TiO2 and Au/TiO2-Al2O3 sol-gel prepared catalysts. J. Mol. Catal. Chem. 2008, 281, 93–98. [Google Scholar] [CrossRef]
- Kowalska, E.; Abe, R.; Ohtani, B. Visible light-induced photocatalytic reaction of gold-modified titanium(IV) oxide particles: Action spectrum analysis. Chem. Commun. 2009, 2, 241–243. [Google Scholar] [CrossRef]
- Furube, A.; Du, L.; Hara, K.; Katoh, R.; Tachiya, M. Ulltrafast plasmon-induced electron transfer from gold nanodots into TiO2 nanoparticles. J. Am. Chem. Soc. 2007, 129, 14852–14853. [Google Scholar] [CrossRef] [PubMed]
- Sakai, N.; Fujiwara, Y.; Arai, M.; Yu, K.; Tatsuma, T. Electrodeposition of gold nanoparticles on ITO: Control of morphology and plasmon resonance-based absorption and scattering. J. Electroanal. Chem. 2009, 628, 7–15. [Google Scholar] [CrossRef]
- Sakai, N.; Fujiwara, Y.; Takahashi, Y.; Tatsuma, T. Plasmon-resonance-based generation of cathodic photocurrent at electrodeposited gold nanoparticles coated with TiO2 films. Chem. Phys. Chem. 2009, 10, 766–769. [Google Scholar] [CrossRef] [PubMed]
- Caretti, I.; Keulemans, M.; Verbruggen, S.W.; Lenaerts, S.; Van Doorslaer, S. Light-induced processes in plasmonic gold/TiO2 photocatalysts studied by electron paramagnetic resonance. Top. Catal. 2015, 58, 776–782. [Google Scholar] [CrossRef]
- Priebe, J.B.; Radnik, J.; Lennox, A.J.J.; Pohl, M.M.; Karnahl, M.; Hollmann, D.; Grabow, K.; Bentrup, U.; Junge, H.; Beller, M.; et al. Solar hydrogen production by plasmonic Au-TiO2 catalysts: Impact of synthesis protocol and TiO2 phase on charge transfer efficiency and H2 evolution rates. ACS Catal. 2015, 5, 2137–2148. [Google Scholar] [CrossRef]
- Mendez-Medrano, M.G.; Kowalska, E.; Lehoux, A.; Herissan, A.; Ohtani, B.; Rau, S.; Colbeau-Justin, C.; Rodriguez-Lopez, J.L.; Remita, H. Surface modification of TiO2 with Au nanoclusters for efficient water treatment and hydrogen generation under visible light. J. Phys. Chem. 2016, 120, 25010–25022. [Google Scholar] [CrossRef]
- Wei, Z.; Janczarek, M.; Endo, M.; Colbeau-Justin, C.; Ohtani, B.; Kowalska, E. Silver-modified octahedral anatase particles as plasmonic photocatalyst. Catal. Today 2018, 310, 19–25. [Google Scholar] [CrossRef]
- Liu, Z.; Hou, W.; Pavaskar, P.; Aykol, M.; Cronin, S.B. Plasmon resonance enhancement of photocatalytic water splitting under visible illumination. Nano Lett. 2011, 11, 1111–1116. [Google Scholar] [CrossRef]
- Bouhadoun, S.; Guillard, C.; Dapozze, F.; Singh, S.; Amans, D.; Boucle, J.; Herlin-Boime, N. One step synthesis of N-doped and Au-loaded TiO2 nanoparticles by laser pyrolysis: Application in photocatalysis. Appl. Catal. Environ. 2015, 174, 367–375. [Google Scholar] [CrossRef]
- Valenti, M.; Dolat, D.; Biskos, G.; Schmidt-Ott, A.; Smith, W.A. Enhancement of the photoelectrochemical performance of CuWO4 thin films for solar water splitting by plasmonic nanoparticle functionalization. J. Phys. Chem. 2015, 119, 2096–2104. [Google Scholar] [CrossRef]
- Hou, W.; Liu, Z.; Pavaskar, P.; Hsuan Hung, W.; Cronin, S.B. Plasmonic enhancement of photocatalytic decomposition of methyl orange under visible light. J. Catal. 2011, 277, 149–153. [Google Scholar] [CrossRef]
- Seh, Z.W.; Liu, S.W.; Low, M.; Zhang, S.-Y.; Liu, Z.; Mlayah, A.; Han, M.-Y. Janus Au-TiO2 photocatalysts with strong localization of plasmonic near fields for efficient visible-light hydrogen generation. Adv. Mater. 2012, 24, 2310–2314. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhu, H.-Y.; Zhao, J.-C.; Zheng, Z.-F.; Gao, X.-P. Visible-light-driven oxidation of organic contaminants in air with gold nanoparticle catalysts on oxide supports. Angew. Chem. Int. Ed. 2008, 47, 5353–5356. [Google Scholar] [CrossRef] [PubMed]
- Son, M.S.; Im, J.E.; Wang, K.K.; Oh, S.L.; Kim, Y.R.; Yoo, K.H. Surface plasmon enhanced photoconductance and single electron effects in mesoporous titania nanofibers loaded with gold nanoparticles. Appl. Phys. Lett. 2010, 96, 023115. [Google Scholar] [CrossRef]
- Mukherjee, S.; Libisch, F.; Large, N.; Neumann, O.; Brown, L.V.; Cheng, J.; Lassiter, J.B.; Carter, E.A.; Nordlander, P.; Halas, N.J. Hot electrons do the impossible: Plasmon-induced dissociation of H2 on Au. Nano Lett. 2013, 13, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Kominami, H.; Tanaka, A.; Hashimoto, K. Gold nanoparticles supported on cerium(IV) oxide powder for mineralization of organic acids in aqueous suspensions under irradiation of visible light of λ = 530 nm. Appl. Catal. Gen. 2011, 397, 121–126. [Google Scholar] [CrossRef]
- Nishijima, Y.; Ueno, K.; Yokata, Y.; Murakoshi, K.; Misawa, H. Plasmon-assisted photocurrent generation from visible to near-infrared wavelength using a Au-nanorods/TiO2 electrode. J. Phys. Chem. Lett. 2010, 1, 2031–2036. [Google Scholar] [CrossRef]
- Kowalska, E.; Mahaney, O.O.P.; Abe, R.; Ohtani, B. Visible-light-induced photocatalysis through surface plasmon excitation of gold on titania surfaces. Phys. Chem. Chem. Phys. 2010, 12, 2344–2355. [Google Scholar] [CrossRef] [Green Version]
- Bian, Z.F.; Tachikawa, T.; Zhang, P.; Fujitsuka, M.; Majima, T. Au/TiO2 superstructure-based plasmonic photocatalysts exhibiting efficient charge separation and unprecedented activity. J. Am. Chem. Soc. 2014, 136, 458–465. [Google Scholar] [CrossRef]
- Murakami, N.; Kurihara, Y.; Tsubota, T.; Ohno, T. Shape-controlled anatase titanium(IV) oxide particles prepared by hydrothermal treatment of peroxo titanic acid in the presence of polyvinyl alcohol. J. Phys. Chem. 2009, 113, 3062–3069. [Google Scholar] [CrossRef] [Green Version]
- Wei, Z.; Janczarek, M.; Endo, M.; Wang, K.L.; Balcytis, A.; Nitta, A.; Mendez-Medrano, M.G.; Colbeau-Justin, C.; Juodkazis, S.; Ohtani, B.; et al. Noble metal-modified faceted anatase titania photocatalysts: Octahedron versus decahedron. Appl. Catal. Environ. 2018, 237, 574–587. [Google Scholar] [CrossRef]
- Wei, Z.; Kowalska, E.; Verrett, J.; Colbeau-Justin, C.; Remita, H.; Ohtani, B. Morphology-dependent photocatalytic activity of octahedral anatase particles prepared by ultrasonication-hydrothermal reaction of titanates. Nanoscale 2015, 7, 12392–12404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leong, K.H.; Chu, H.Y.; Ibrahim, S.; Saravanan, P. Palladium nanoparticles anchored to anatase TiO2 for enhanced surface plasmon resonance-stimulated, visible-light-driven photocatalytic activity. Beilstein J. Nanotechnol. 2015, 6, 428–437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zielinska-Jurek, A.; Wei, Z.; Wysocka, I.; Szweda, P.; Kowalska, E. The effect of nanoparticles size on photocatalytic and antimicrobial properties of Ag-Pt/TiO2 photocatalysts. Appl. Surf. Sci. 2015, 353, 317–325. [Google Scholar] [CrossRef] [Green Version]
- DeSario, P.A.; Pietron, J.J.; Brintlinger, T.H.; McEntee, M.; Parker, J.F.; Baturina, O.; Stroud, R.M.; Rolison, D.R. Oxidation-stable plasmonic copper nanoparticles in photocatalytic TiO2 nanoarchitectures. Nanoscale 2017, 9, 11720–11729. [Google Scholar] [CrossRef] [Green Version]
- Link, S.; El-Sayed, M.A. Spectral properties and relaxation dynamics of surface plasmon electronic oscillations in gold and silver nanodots and nanorods. J. Phys. Chem. B 1999, 103, 8410–8426. [Google Scholar] [CrossRef]
- Muniz-Miranda, M.; Gellini, C.; Simonelli, A.; Tiberi, M.; Giammanco, F.; Giorgetti, E. Characterization of Copper nanoparticles obtained by laser ablation in liquids. Appl. Phys. Mater. 2013, 110, 829–833. [Google Scholar] [CrossRef]
- Nilius, N.; Ernst, N.; Freund, H. On energy transfer processes at cluster-oxide interfaces: Silver on titania. Chem. Phys. Lett. 2001, 349, 351–357. [Google Scholar] [CrossRef]
- Xia, Y.N.; Xiong, Y.J.; Lim, B.; Skrabalak, S.E. Shape-controlled synthesis of metal nanocrystals: Simple chemistry meets complex physics? Angew. Chem. Int. Ed. 2009, 48, 60–103. [Google Scholar] [CrossRef]
- Yablonovitch, E. Photonic band-gap structures. J. Opt. Soc. Am. 1993, 10, 283–295. [Google Scholar] [CrossRef]
- Lopez, C. Materials aspects of photonic crystals. Adv. Mater. 2003, 15, 1679–1704. [Google Scholar] [CrossRef]
- Kim, W.T.; Choi, W.Y. Fabrication of TiO2 photonic crystal by anodic oxidation and their optical sensing properties. Sens. Actuators A Phys. 2017, 260, 178–184. [Google Scholar] [CrossRef]
- Chiarello, G.L.; Zuliani, A.; Ceresoli, D.; Martinazzo, R.; Selli, E. Exploiting the photonic crystal properties of TiO2 nanotube arrays to enhance photocatalytic hydrogen production. ACS Catal. 2016, 6, 1345–1353. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Yang, X.L.; Hedhili, M.N.; Ahmed, E.; Shi, L.; Wang, P. Microwave-assisted self-doping of TiO2 photonic crystals for efficient photoelectrochemical water splitting. ACS Appl. Mater. Interfaces 2014, 6, 691–696. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.H.; Wu, H.J. Multiple band light trapping in ultraviolet, visible and near infrared regions with TiO2 based photonic materials. Chem. Commun. 2014, 50, 14179–14182. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.Z.; Xin, Y.M.; Wu, W.L.; Fu, B.H.; Zhang, Z.H. Phosphorus cation doping: A new strategy for boosting photoelectrochemical performance on TiO2 nanotube photonic crystals. ACS Appl. Mater. Interfaces 2016, 8, 30972–30979. [Google Scholar] [CrossRef]
- Likodimos, V. Photonic crystal-assisted visible light activated TiO2 photocatalysis. Appl. Catal. Environ. 2018, 230, 269–303. [Google Scholar] [CrossRef]
- Li, X.; Yu, J.G.; Jaroniec, M. Hierarchical photocatalysts. Chem. Soc. Rev. 2016, 45, 2603–2636. [Google Scholar] [CrossRef]
- Chiang, C.C.; Tuyen, L.D.; Ren, C.R.; Chau, L.K.; Wu, C.Y.; Huang, P.J.; Hsu, C.C. Fabrication of titania inverse opals by multi-cycle dip-infiltration for optical sensing. Photonics Nanostruct. 2016, 19, 48–54. [Google Scholar] [CrossRef]
- Lu, X.Y.; Zhu, Y.; Cen, T.Z.; Jiang, L. Centimeter-scale colloidal crystal belts via robust self-assembly strategy. Langmuir 2012, 28, 9341–9346. [Google Scholar] [CrossRef]
- Jiang, P.; Bertone, J.F.; Hwang, K.S.; Colvin, V.L. Single-crystal colloidal multilayers of controlled thickness. Chem. Mater. 1999, 11, 2132–2140. [Google Scholar] [CrossRef]
- Li, H.; Vienneau, G.; Jones, M.; Subramanian, B.; Robichaud, J.; Djaoued, Y. Crack-free 2D-inverse opal anatase TiO2 films on rigid and flexible transparent conducting substrates: Low temperature large area fabrication and electrochromic properties. J. Mater. Chem. 2014, 2, 7804–7810. [Google Scholar] [CrossRef]
- Mayoral, R.; Requena, J.; Moya, J.S.; Lopez, C.; Cintas, A.; Miguez, H.; Meseguer, F.; Vazquez, L.; Holgado, M.; Blanco, A. 3D long-range ordering in an SiO2 submicrometer-sphere sintered superstructure. Adv. Mater. 1997, 9, 257–260. [Google Scholar] [CrossRef] [Green Version]
- Kubrin, R.; Pasquarelli, R.M.; Waleczek, M.; Lee, H.S.; Zierold, R.; do Rosario, J.J.; Dyachenko, P.N.; Moreno, J.M.M.; Petrov, A.Y.; Janssen, R.; et al. Bottom-up fabrication of multilayer stacks of 3D photonic crystals from titanium dioxide. ACS Appl. Mater. Interfaces 2016, 8, 10466–10476. [Google Scholar] [CrossRef] [PubMed]
- Curti, M.; Robledo, G.L.; Claro, P.C.D.; Ubogui, J.H.; Mendive, C.B. Characterization of titania inverse opals prepared by two distinct infiltration approaches. Mater. Res. Bull. 2018, 101, 12–19. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, K.; Su, F.Y.; Cai, Z.Y.; Liu, J.X.; Wu, X.W.; He, H.L.; Yin, Z.; Wang, L.H.; Wang, B.; et al. Electrically switchable photonic crystals based on liquid-crystal-infiltrated TiO2-inverse opals. Opt. Express 2019, 27, 15391–15398. [Google Scholar] [CrossRef]
- Kim, K.; Thiyagarajan, P.; Ahn, H.J.; Kim, S.I.; Jang, J.H. Optimization for visible light photocatalytic water splitting: Gold-coated and surface-textured TiO2 inverse opal nano-networks. Nanoscale 2013, 5, 6254–6260. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Q.; Dong, P.; Liu, L.X.; Cheng, B.Y. Study on the sedimentation self-assembly of colloidal SiO2 particles under gravitational field. Colloids Surf. 2005, 253, 169–174. [Google Scholar] [CrossRef]
- Hua, C.X.; Xu, H.B.; Zhang, P.P.; Chen, X.Y.; Lu, Y.Y.; Gan, Y.; Zhao, J.P.; Li, Y. Process optimization and optical properties of colloidal self-assembly via refrigerated centrifugation. Colloid. Polym. Sci. 2017, 295, 1655–1662. [Google Scholar] [CrossRef]
- Miguez, H.; Meseguer, F.; Lopez, C.; Blanco, A.; Moya, J.S.; Requena, J.; Mifsud, A.; Fornes, V. Control of the photonic crystal properties of fcc-packed submicrometer SiO2 spheres by sintering. Adv. Mater. 1998, 10, 480–483. [Google Scholar] [CrossRef]
- Jovic, V.; Idriss, H.; Waterhouse, G.I.N. Slow photon amplification of gas-phase ethanol photo-oxidation in titania inverse opal photonic crystals. Chem. Phys. 2016, 479, 109–121. [Google Scholar] [CrossRef]
- Cheng, C.W.; Karuturi, S.K.; Liu, L.J.; Liu, J.P.; Li, H.X.; Su, L.T.; Tok, A.I.Y.; Fan, H.J. Quantum-dot-sensitized TiO2 inverse opals for photoelectrochemical hydrogen generation. Small 2012, 8, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.J.; Karuturi, S.K.; Su, L.T.; Tok, A.I.Y. TiO2 inverse-opal electrode fabricated by atomic layer deposition for dye-sensitized solar cell applications. Energy Environ. Sci. 2011, 4, 209–215. [Google Scholar] [CrossRef]
- Li, X.H.; Wu, Y.; Shen, Y.H.; Sun, Y.; Yang, Y.; Xie, A.J. A novel bifunctional Ni-doped TiO2 inverse opal with enhanced SERS performance and excellent photocatalytic activity. Appl. Surf. Sci. 2018, 427, 739–744. [Google Scholar] [CrossRef]
- Moon, J.H.; Cho, Y.S.; Yang, S.M. Room temperature chemical vapor deposition for fabrication of titania inverse opals: Fabrication, morphology analysis and optical characterization. Korean Chem. Soc. 2009, 30, 2245–2248. [Google Scholar]
- Curti, M.; Mendive, C.B.; Grela, M.A.; Bahnemann, D.W. Stopband tuning of TiO2 inverse opals for slow photon absorption. Mater. Res. Bull. 2017, 91, 155–165. [Google Scholar] [CrossRef]
- Sordello, F.; Duca, C.; Maurino, V.; Minero, C. Photocatalytic metamaterials: TiO2 inverse opals. Chem. Commun. 2011, 47, 6147–6149. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Liu, J.; Jin, J.; Wang, C.; Huang, S.Z.; Deng, Z.; Li, Y.; Su, B.L. Probing significant light absorption enhancement of titania inverse opal films for highly exalted photocatalytic degradation of dye pollutants. Appl. Catal. Environ. 2014, 150, 411–420. [Google Scholar] [CrossRef]
- Lu, Y.; Yu, H.T.; Chen, S.; Quan, X.; Zhao, H.M. Integrating plasmonic nanoparticles with TiO2 photonic crystal for enhancement of visible-light-driven photocatalysis. Environ. Sci. Technol. 2012, 46, 1724–1730. [Google Scholar] [CrossRef]
- Curti, M.; Zvitco, G.; Grela, M.A.; Mendive, C.B. Angle dependence in slow photon photocatalysis using TiO2 inverse opals. Chem. Phys. 2018, 502, 33–38. [Google Scholar] [CrossRef]
- Zhao, Y.X.; Yang, B.F.; Xu, J.; Fu, Z.P.; Wu, M.; Li, F. Facile synthesis of Ag nanoparticles supported on TiO2 inverse opal with enhanced visible-light photocatalytic activity. Thin Solid Films 2012, 520, 3515–3522. [Google Scholar] [CrossRef]
- Srinivasan, M.; White, T. Degradation of methylene blue by three-dimensionally ordered macroporous titania. Environ. Sci. Technol. 2007, 41, 4405–4409. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Wang, J.; Wang, X.; Xu, H.; Yuan, S.; Zhang, Q.; Zhang, M. Preparation of inverse opal titanium dioxide for photocatalytic performance research. Opt. Mater. 2019, 96, 109287. [Google Scholar] [CrossRef]
- Rahul, T.K.; Sandhyarani, N. Nitrogen-fluorine co-doped titania inverse opals for enhanced solar light driven photocatalysis. Nanoscale 2015, 7, 18259–18270. [Google Scholar] [CrossRef]
- Cai, J.M.; Wu, M.Q.; Wang, Y.T.; Zhang, H.; Meng, M.; Tian, Y.; Li, X.G.; Zhang, J.; Zheng, L.R.; Gong, J.L. Synergetic enhancement of light harvesting and charge separation over surface-disorder-engineered TiO2 photonic crystals. Chem 2017, 2, 877–892. [Google Scholar] [CrossRef] [Green Version]
- Toumazatou, A.; Arfanis, M.K.; Pantazopoulos, P.-A.; Kontos, A.G.; Falaras, P.; Stefanou, N.; Likodimos, V. Slow-photon enhancement of dye sensitized TiO2 photocatalysis. Mater. Lett. 2017, 197, 123–126. [Google Scholar] [CrossRef]
- Chen, Z.; Fang, L.; Dong, W.; Zheng, F.; Shen, M.; Wang, J. Inverse opal structured Ag/TiO2 plasmonic photocatalyst prepared by pulsed current deposition and its enhanced visible light photocatalytic activity. J. Mater. Chem. 2014, 2, 824–832. [Google Scholar] [CrossRef]
- Lim, S.Y.; Law, C.S.; Liu, L.; Markovis, M.; Abell, A.D.; Santos, A. Integrating surface plasmon resonance and slow photon effects in nanoporous anodis alumina photonic crystals for photocatalysis. Catal. Sci. Technol. 2019, 9, 3158–3176. [Google Scholar] [CrossRef]
- Ye, J.; He, J.H.; Wang, S.; Zhou, X.J.; Zhang, Y.; Liu, G.; Yang, Y.F. Nickel-loaded black TiO2 with inverse opal structure for photocatalytic reduction of CO2 under visible light. Sep. Purif. Technol. 2019, 220, 8–15. [Google Scholar] [CrossRef]
- Wang, X.Y.; Li, J.; Gao, X.N.; Shen, Y.H.; Xie, A.J. Ordered CdSe-sensitized TiO2 inverse opal film as multifunctional surface-enhanced Raman scattering substrate. Appl. Surf. Sci. 2019, 463, 357–362. [Google Scholar] [CrossRef]
- Zheng, X.Z.; Li, D.Z.; Li, X.F.; Chen, J.; Cao, C.S.; Fang, J.L.; Wang, J.B.; He, Y.H.; Zheng, Y. Construction of ZnO/TiO2 photonic crystal heterostructures for enhanced photocatalytic properties. Appl. Catal. Environ. 2015, 168, 408–415. [Google Scholar] [CrossRef]
- Lee, H.; Jeon, T.Y.; Lee, S.Y.; Lee, S.Y.; Kim, S.H. Designing multicolor micropatterns of inverse opals with photonic bandgap and surface plasmon resonance. Adv. Funct. Mater. 2018, 28, 1706664. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Zheng, Y.H.; Liu, X.; Lu, W.; Dai, J.Y.; Lei, D.Y.; MacFarlane, D.R. Hierarchical porous plasmonic metamaterials for reproducible ultrasensitive surface-enhanced Raman spectroscopy. Adv. Mater. 2015, 27, 1090–1096. [Google Scholar] [CrossRef] [PubMed]
- Rahul, T.K.; Sandhyarani, N. Plasmonic and photonic effects on hydrogen evolution over chemically modified titania inverse opals. Chemnanomat 2018, 4, 642–648. [Google Scholar] [CrossRef]
- Jiao, J.Q.; Wei, Y.C.; Chi, K.B.; Zhao, Z.; Duan, A.J.; Liu, J.; Jiang, G.Y.; Wang, Y.J.; Wang, X.L.; Han, C.C.; et al. Platinum nanoparticles supported on TiO2 photonic crystals as highly active photocatalyst for the reduction of CO2 in the presence of water. Energy Technol. 2017, 5, 877–883. [Google Scholar] [CrossRef] [Green Version]
- Ding, B.Y.; Pemble, M.E.; Korovin, A.V.; Peschel, U.; Romanov, S.G. Three-dimensional photonic crystals with an active surface: Gold film terminated opals. Phys. Rev. 2010, 82, 035119. [Google Scholar] [CrossRef]
- Paterno, G.M.; Moscardi, L.; Donini, S.; Ariodanti, D.; Kriegel, I.; Zani, M.; Parisini, E.; Scotognella, F.; Lanzani, G. Hybrid one-dimensional plasmonic-photonic crystals for optical detection of bacterial contaminants. J. Phys. Chem. Lett. 2019, 10, 4980–4986. [Google Scholar] [CrossRef]
- Zhao, H.; Hu, Z.Y.; Liu, J.; Li, Y.; Wu, M.; Van Tendeloo, G.; Su, B.L. Blue-edge slow photons promoting visible-light hydrogen production on gradient ternary 3DOM TiO2-Au-CdS photonic crystals. Nano Energy 2018, 47, 266–274. [Google Scholar] [CrossRef]
- Erola, M.O.A.; Philip, A.; Ahmed, T.; Suvanto, S.; Pakkanen, T.T. Fabrication of Au- and Ag-SiO2 inverse opals having both localized surface plasmon resonance and Bragg diffraction. J. Solid State Chem. 2015, 230, 209–217. [Google Scholar] [CrossRef]
- Chen, J.I.L.; Loso, E.; Ebrahim, N.; Ozin, G.A. Synergy of slow photon and chemically amplified photochemistry in platinum nanocluster-loaded inverse titania opals. J. Am. Chem. Soc. 2008, 130, 5420–5421. [Google Scholar] [CrossRef]
- Sanchez-Garcia, L.; Tserkezis, C.; Ramirez, M.O.; Molina, P.; Carvajal, J.J.; Aguilo, M.; Diaz, F.; Aizpurua, J.; Bausa, L.E. Plasmonic enhancement of second harmonic generation from nonlinear RbTiOPO4 crystals by aggregates of silver nanostructures. Opt. Express 2016, 24, 8491–8500. [Google Scholar] [CrossRef] [Green Version]
- Meng, S.G.; Li, D.Z.; Fu, X.L.; Fu, X.Z. Integrating photonic bandgaps with surface plasmon resonance for the enhancement of visible-light photocatalytic performance. J. Mater. Chem. 2015, 3, 23501–23511. [Google Scholar] [CrossRef]
- Zeng, S.; Vahidzadeh, E.; VanEssen, C.G.; Kar, P.; Kisslinger, R.; Goswami, A.; Zhang, Y.; Mandi, N.; Riddell, S.; Kobryn, A.E.; et al. Optical control of selectivity of high rate CO2 photoreduction via interbandor hot electron Z-scheme reaction pathways in Au-TiO2 plasmonic photonic crystal photocatalyst. Appl. Catal. Environ. 2020, 267, 118644. [Google Scholar] [CrossRef]
- Fang, L.; Nan, F.; Yang, Y.; Cao, D.W. Enhanced photoelectrochemical and photocatalytic activity in visible-light-driven Ag/BiVO4 inverse opals. Appl. Phys. Lett. 2016, 108, 093902. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, L.; Hedhili, M.N.; Zhang, H.; Wang, P. Plasmonic gold nanocrystals coupled with photonic crystal seamlessly on TiO2 nanotube photoelectrodes for efficient visible light photoelectrochemical water splitting. Nano Lett. 2013, 13, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, Y.; Lee, S.T.; Yang, S.H.; Kang, Z.H. Coupling surface plasmon resonance of gold nanoparticles with slow-photon-effect of TiO2 photonic crystals for synergistically enhanced photoelectrochemical water splitting. Energy Environ. Sci. 2014, 7, 1409–1419. [Google Scholar] [CrossRef]
- Wang, Y.F.; Xiong, D.B.; Zhang, W.; Su, H.L.; Liu, Q.L.; Gu, J.J.; Zhu, S.M.; Zhang, D. Surface plasmon resonance of gold nanocrystals coupled with slow-photon-effect of biomorphic TiO2 photonic crystals for enhanced photocatalysis under visible-light. Catal. Today 2016, 274, 15–21. [Google Scholar] [CrossRef]
- Huo, J.W.; Yuan, C.; Wang, Y. Nanocomposites of three-dimensionally ordered porous TiO2 decorated with Pt and reduced graphene oxide for the visible-light photocatalytic degradation of waterborne pollutants. ACS Appl. Nano Mater. 2019, 2, 2713–2724. [Google Scholar] [CrossRef]
- Temerov, F.; Ankudze, B.; Saarinen, J.J. TiO2 inverse opal structures with facile decoration of precious metal nanoparticles for enhanced photocatalytic activity. Mater. Chem. Phys. 2020, 242, 122471. [Google Scholar] [CrossRef]
- Zhang, S.S.; Peng, B.Y.; Yang, S.Y.; Wang, H.G.; Yu, H.; Fang, Y.P.; Peng, F. Non-noble metal copper nanoparticles-decorated TiO2 nanotube arrays with plasmon-enhanced photocatalytic hydrogen evolution under visible light. Int. J. Hydrog. Energy 2015, 40, 303–310. [Google Scholar] [CrossRef]
- Zhang, L.W.; Lin, C.Y.; Valev, V.K.; Reisner, E.; Steiner, U.; Baumberg, J.J. Plasmonic enhancement in BiVO4 photonic crystals for efficient water splitting. Small 2014, 10, 3970–3978. [Google Scholar] [CrossRef] [Green Version]
- Dinh, C.T.; Yen, H.; Kleitz, F.; Do, T.O. Three-dimensional ordered assembly of thin-shell Au/TiO2 hollow nanospheres for enhanced visible-light-driven photocatalysis. Angew. Chem. Int. Ed. 2014, 53, 6618–6623. [Google Scholar] [CrossRef] [PubMed]
- Alessandri, I.; Ferroni, M. Exploiting optothermal conversion for nanofabrication: Site-selective generation of Au/TiO2 inverse opals. J. Mater. Chem. 2009, 19, 7990–7994. [Google Scholar] [CrossRef]
- Wei, Z.; Endo, M.; Wang, K.; Charbit, E.; Markowska-Szczupak, A.; Ohtani, B.; Kowalska, E. Noble metal-modified octahedral anatase titania particles with enhanced activity for decomposition of chemical and microbiological pollutants. Chem. Eng. J. 2017, 318, 121–134. [Google Scholar] [CrossRef] [PubMed]
- Raja-Mogan, T.; Lehoux, A.; Takashima, M.; Kowalska, E.; Ohtani, B. A triply wavelength-tuned visible light-responsive photocatalyst: Matching of LED, PBG and LSPR. 2020. under preparation for submission. [Google Scholar]









| Plasmonic Based PCs | Plasmonic NPs Loading/Deposition Methods | Photocatalytic Tests | Findings | References |
|---|---|---|---|---|
| Ptx/PC-TiO2 | gas bubbling-assisted membrane reduction | CO2 reduction; λ = 320–780 nm; I | 3.2 times higher activity * | [107] |
| TiO2 PC/Au NPs | in situ hydrothermal reduction | degradation of 2,4- dichlorophenol; λ > 420 nm; II | PBG and SPR overlapping | [91] |
| AgTIO | chemical route | MB degradation; λ = 254 nm and λ = 400–760 nm; III | SPR and PBG enhancing activity | [93] |
| Au-TiO2-NAA-DBRs | sputter coating | MB degradation; III | enhanced activity | [100] |
| TiO2-Au-CdS | immersion and chemical bath deposition | H2 production; λ > 420 nm; IV | enhanced H2 generation at blue-edge PBG | [110] |
| i-Pt-TiO2-o film | photodeposition | degradation of acid orange; λ > 400 nm; I | 4-fold enhancement * | [112] |
| Au/ZnO-PCs | magnetron sputtering | photodegradation of RhB; λ > 420 nm; II | 24.8-fold higher than commercial ZnO | [114] |
| Au-PCTNTs | magnetron sputtering | CO2 photoreduction; λ > 400 nm; III | high selectivity of methane generation | [115] |
| Ag/BiVO4 | electrodeposition | MB degradation; λ > 420 nm; III | enhanced activity | [116] |
| Au/TNTs | photodeposition | IPCE (400–700 nm); photocurrent ≥ 420 nm; water splitting; II | PBG matching with LSPR—enhanced activity | [117] |
| Au/TiO2 photoanode | facile ionic layer adsorption and thermal-reduction | PEC water splitting; λ > 420 nm; II | 0.71% of solar energy conversion | [118] |
| Au/TiO2 PC | chemical route | MO degradation λ > 420 nm; III | 7-fold increase * | [119] |
| rGO/Pt/3DOM TiO2 | dropwise and thermal reduction | MO degradation; λ > 420 nm; I | 4-fold increase * | [120] |
| TiO2-IO Au/AgNPs film | immersion | acetylene mineralization; λ = 365 nm; I | enhanced activity * | [121] |
| Cu/TNTs | pulsed electrochemical deposition | H2 production λ > 400 nm; II | enhanced activity | [122] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raja-Mogan, T.; Ohtani, B.; Kowalska, E. Photonic Crystals for Plasmonic Photocatalysis. Catalysts 2020, 10, 827. https://doi.org/10.3390/catal10080827
Raja-Mogan T, Ohtani B, Kowalska E. Photonic Crystals for Plasmonic Photocatalysis. Catalysts. 2020; 10(8):827. https://doi.org/10.3390/catal10080827
Chicago/Turabian StyleRaja-Mogan, Tharishinny, Bunsho Ohtani, and Ewa Kowalska. 2020. "Photonic Crystals for Plasmonic Photocatalysis" Catalysts 10, no. 8: 827. https://doi.org/10.3390/catal10080827