Biomass-Derived Nitrogen-Doped Porous Carbon for Highly Efficient Ambient Electro-Synthesis of NH3
Abstract
:1. Introduction
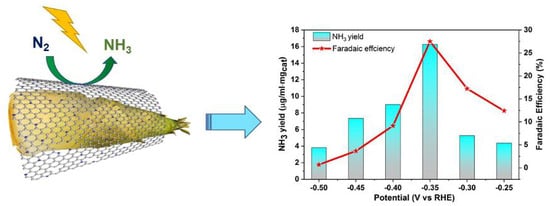
2. Results and Discussion
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Robert, F.S. Chemistry. New recipe produces ammonia from air, water, and sunlight. Science 2014, 345, 610. [Google Scholar]
- Wang, W.T.; Herreros, J.M.; Tsolakis, A.; York, A.P.E. Ammonia as hydrogen carrier for transportation; investigation of the ammonia exhaust gas fuel reforming. Int. J. Hydrogen Energy 2013, 38, 9907–9917. [Google Scholar] [CrossRef] [Green Version]
- Van der Ham, C.J.M.; Koper, M.T.M.; Hetterscheid, D.G.H. Challenges in reduction of dinitrogen by proton and electron transfer. Chem. Soc. Rev. 2014, 43, 5183–5191. [Google Scholar] [CrossRef] [PubMed]
- Kandemir, T.; Schuster, M.E.; Senyshyn, A.; Behrens, M.; Schlögl, R. The Haber–Bosch process revisited: On the real structure and stability of “ammonia iron” under working conditions. Angew. Chem. Int. Ed. 2013, 52, 12723–12726. [Google Scholar] [CrossRef]
- Broda, H.; Tuczek, F. Catalytic ammonia synthesis in homogeneous solution—Biomimetic at last? Angew. Chem. Int. Ed. 2014, 53, 632–634. [Google Scholar] [CrossRef]
- Chen, X.Z.; Li, N.; Kong, Z.Z.; Ong, W.J.; Zhao, X.J. Photocatalytic fixation of nitrogen to ammonia: State-of-the-art advancements and future prospects. Mater. Horiz. 2018, 5, 9–27. [Google Scholar] [CrossRef]
- Chen, X.R.; Guo, Y.T.; Du, X.C.; Zeng, Y.S.; Chu, J.W.; Gong, C.H.; Huang, J.W.; Fan, C.; Wang, X.F.; Xiong, J. Atomic structure modification for electrochemical nitrogen reduction to ammonia. Adv. Energy Mater. 2019, 10, 1903172. [Google Scholar] [CrossRef]
- Zhao, Y.X.; Shi, R.; Bian, X.A.; Zhou, C.; Zhao, Y.F.; Zhang, S.; Wu, F.; Waterhouse, G.I.N.; Wu, L.Z.; Tung, C.H.; et al. Ammonia detection methods in photocatalytic and electrocatalytic experiments: How to improve the reliability of NH3 production rates? Adv. Sci. 2019, 6, 1802109. [Google Scholar] [CrossRef] [Green Version]
- Andersen, S.Z.; Čolić, V.; Yang, S.G.; Schwalbe, J.A.; Nielander, A.C.; MacEnaney, J.M.; Rasmussen, K.E.; Baker, J.G.; Singh, A.R.; Rohr, B.A.; et al. A rigorous electrochemical ammonia synthesis protocol with quantitative isotope measurements. Nature 2019, 570, 504–508. [Google Scholar] [CrossRef] [Green Version]
- Suryanto, B.H.R.; Du, H.L.; Wang, D.B.; Chen, J.; Simonov, A.N.; MacFarlane, D.R. Challenges and prospects in the catalysis of electroreduction of nitrogen to ammonia. Nat. Catal. 2019, 2, 290–296. [Google Scholar] [CrossRef] [Green Version]
- Xu, B.; Liu, Z.C.; Qiu, W.B.; Liu, Q.; Sun, X.P.; Cui, G.W.; Wu, Y.P.; Xiong, X.L. La2O3 nanoplate: An efficient electrocatalyst for artificial N2 fixation to NH3 with excellent selectivity at ambient condition. Electrochim. Acta 2019, 298, 106–111. [Google Scholar] [CrossRef]
- Zang, W.J.; Yang, T.; Zou, H.Y.; Xi, S.B.; Zhang, H.; Liu, X.M.; Kou, Z.K.; Du, Y.H.; Feng, Y.P.; Shen, L.; et al. Copper single atoms anchored in porous nitrogen-doped carbon as efficient pH-Universal catalysts for the nitrogen reduction reaction. ACS Catal. 2019, 9, 10166–10173. [Google Scholar] [CrossRef]
- Zhao, S.L.; Lu, X.Y.; Wang, L.Z.; Gale, J.L.; Amal, R. Carbon-based metal-free catalysts for electrocatalytic reduction of nitrogen for synthesis of ammonia at ambient conditions. Adv. Mater. 2019, 31, 1805367. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.T.; Li, P.P.; Wang, H.B.; Zhao, R.B.; Zhou, Q.; Kong, W.H.; Liu, M.L.; Zhang, Y.Y.; Sun, X.P.; Gong, F.F. Biomass-derived oxygen-doped hollow carbon microtubes for electrocatalytic N2-to-NH3 fixation under ambient conditions. Chem. Commun. 2019, 55, 2684–2687. [Google Scholar] [CrossRef]
- Kong, Y.; Li, Y.; Yang, B.; Li, Z.J.; Yao, Y.; Lu, J.G.; Lei, L.C.; Wen, Z.H.; Shao, M.H.; Hou, Y. Boron and nitrogen co-doped porous carbon nanofibers as metal-free electrocatalysts for highly efficient ammonia electrosynthesis. J. Mater. Chem. A 2019, 7, 26272–26278. [Google Scholar] [CrossRef]
- Xia, L.; Yang, J.J.; Wang, H.B.; Zhao, R.B.; Chen, H.Y.; Fang, W.H.; Asiri, A.M.; Xie, F.Y.; Cui, G.L.; Sun, X.P. Sulfur-doped graphene for efficient electrocatalytic N2-to-NH3 fixation. Chem. Commun. 2019, 55, 3371–3374. [Google Scholar] [CrossRef]
- Yu, X.M.; Han, P.; Wei, Z.Z.; Huang, L.S.; Gu, Z.X.; Peng, S.J.; Ma, J.M.; Zheng, G.F. Boron-doped graphene for electrocatalytic N2 reduction. Joule 2018, 2, 1610–1622. [Google Scholar] [CrossRef] [Green Version]
- Yuan, D.; Wei, Z.X.; Han, P.; Yang, C.; Huang, L.S.; Gu, Z.X.; Ding, Y.; Ma, J.M.; Zheng, G.F. Electron distribution tuning of fluorine-doped carbon for ammonia electrosynthesis. J. Mater. Chem. A 2019, 7, 16979–16983. [Google Scholar] [CrossRef]
- Song, P.F.; Wang, H.; Kang, L.; Rang, B.C.; Song, H.H.; Wang, R.M. Electrochemical nitrogen reduction to ammonia at ambient conditions on nitrogen and phosphorus co-doped porous carbon. Chem. Commun. 2019, 55, 687–690. [Google Scholar] [CrossRef]
- Wang, T.T.; Zhao, Q.D.; Fu, Y.Y.; Lei, C.J.; Yang, B.; Li, Z.J.; Lei, L.C.; Wu, G.; Hou, Y. Carbon-Rich nonprecious metal single atom electrocatalysts for CO2 reduction and hydrogen evolution. Small Methods 2019, 3, 1900210. [Google Scholar] [CrossRef]
- Signarvic, R.S.; Degrado, W.F. Metal-binding dependent disruption of membranes by designed helices. J. Am. Chem. Soc. 2009, 131, 3377–3384. [Google Scholar] [CrossRef] [Green Version]
- Zhao, C.J.; Zhang, S.B.; Hu, M.M.; Zhang, X.; Liu, Y.Y.; Li, W.Y.; Chen, C.; Wang, G.Z.; Zhang, H.M.; Zhao, H.J. Ambient electrosynthesis of ammonia on a biomass-derived nitrogen-doped porous carbon electrocatalyst: Contribution of pyridinic nitrogen. ACS Energy Lett. 2019, 4, 377–383. [Google Scholar] [CrossRef]
- Ji, G.J.; Duan, Y.N.; Zhang, S.C.; Fei, B.H.; Chen, X.F.; Yang, Y. Selective semihydrogenation of alkynes catalyzed by Pd nanoparticles immobilized on heteroatom-doped hierarchical porous carbon derived from bamboo shoots. ChemSusChem 2017, 10, 3427–3434. [Google Scholar] [CrossRef]
- Chen, C.; Yang, D.F.; Wang, Y.; Zhou, Y.Y.; Zou, Y.Q.; Li, Y.F.; Wang, S.Y. B-N Pairs enriched defective carbon nanosheets for ammonia synthesis with high efficiency. Small 2019, 15, 1805029. [Google Scholar] [CrossRef]
- Deng, Q.F.; Liu, L.; Lin, X.Z.; Du, G.; Liu, Y.; Yuan, Z.Y. Synthesis and CO2 capture properties of mesoporous carbon nitride materials. Chem. Eng. J. 2012, 203, 63–70. [Google Scholar] [CrossRef]
- Fan, X.; Zhang, L.; Zhang, G.; Shu, Z.; Shi, J. Chitosan derived nitrogen-doped microporous carbons for high performance CO2 capture. Carbon 2013, 61, 423–430. [Google Scholar] [CrossRef]
- Song, T.; Ren, P.; Duan, Y.N.; Wang, Z.Z.; Chen, X.F.; Yang, Y. Cobalt nanocomposites on N-doped hierarchical porous carbon for highly selective formation of anilines and imines from nitroarenes. Green Chem. 2018, 20, 4629–4637. [Google Scholar] [CrossRef]
- Wang, X.Y.; Zhao, Q.D.; Yang, B.; Li, Z.J.; Bo, Z.; Lam, K.H.; Adli, N.M.; Lei, L.C.; Wen, Z.H.; Wu, G.; et al. Emerging nanostructured carbon-based non-precious metal electrocatalysts for selective electrochemical CO2 reduction to CO. J. Mater. Chem. A 2019, 7, 25191–25202. [Google Scholar] [CrossRef]
- Shi, M.M.; Bao, D.; Wulan, B.R.; Li, Y.H.; Zhang, Y.F.; Yan, J.M.; Jiang, Q. Au Sub-Nanoclusters on TiO2 toward highly efficient and selective electrocatalyst for N2 conversion to NH3 at ambient conditions. Adv. Mater. 2017, 29, 1606550. [Google Scholar] [CrossRef]
- Watt, G.W.; Chrisp, J.D. Spectrophotometric method for determination of hydrazine. Anal. Chem. 1952, 24, 2006–2008. [Google Scholar] [CrossRef]
- Zhou, F.L.; Azofra, L.M.; Ali, M.; Kar, M.; Simonov, A.N.; Mcdonnell-Worth, C.; Sun, C.H.; Zhang, X.Y.; Macfarlane, D.R. Electro-synthesis of ammonia from nitrogen at ambient temperature and pressure in ionic liquids. Energy Environ. Sci. 2017, 10, 2516–2520. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Q.; Li, C.; Fan, H.N.; Luo, Q.B.; Liu, H.K.; Dou, S.X. Atomically dispersed metal dimer species with selective catalytic activity for nitrogen electrochemical reduction. J. Mater. Chem. A 2019, 7, 22242–22247. [Google Scholar] [CrossRef]
- Tang, C.; Qiao, S.Z. How to explore ambient electrocatalytic nitrogen reduction reliably and insightfully. Chem. Soc. Rev. 2019, 48, 3166–3180. [Google Scholar] [CrossRef]
- Wang, Z.H.; Hu, X.; Liu, Z.Z.; Zou, G.J.; Wang, G.N.; Zhang, K. Recent developments in polymeric carbon nitride-derived photocatalysts and electrocatalysts for nitrogen fixation. ACS Catal. 2019, 9, 10260–10278. [Google Scholar] [CrossRef]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Q.; Chen, X.; Yang, Y. Biomass-Derived Nitrogen-Doped Porous Carbon for Highly Efficient Ambient Electro-Synthesis of NH3. Catalysts 2020, 10, 353. https://doi.org/10.3390/catal10030353
Li Q, Chen X, Yang Y. Biomass-Derived Nitrogen-Doped Porous Carbon for Highly Efficient Ambient Electro-Synthesis of NH3. Catalysts. 2020; 10(3):353. https://doi.org/10.3390/catal10030353
Chicago/Turabian StyleLi, Qinglin, Xiufang Chen, and Yong Yang. 2020. "Biomass-Derived Nitrogen-Doped Porous Carbon for Highly Efficient Ambient Electro-Synthesis of NH3" Catalysts 10, no. 3: 353. https://doi.org/10.3390/catal10030353