Highly Active Trifloaluminate Ionic Liquids as Recyclable Catalysts for Green Oxidation of 2,3,6-Trimethylphenol to Trimethyl-1,4-Benzoquinone
Abstract
:1. Introduction
2. Results and Discussion
2.1. Homogeneous System with Trifloaluminate Ionic Liquids
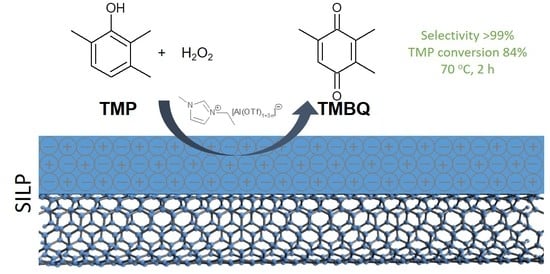
2.2. Heterogeneous System with Trifloaluminate Ionic Liquids
3. Materials and Methods
3.1. Materials
3.2. Experimental Procedures
3.2.1. Homogenous Catalyst
3.2.2. Heterogeneous Catalyst
3.2.3. Synthesis of 2,3,6-Trimethyl-1,4-Benzoquinone
3.3. Analyses
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Suttie, J.W. Handbook of Vitamins, 3rd ed.; Rucker, R.B., Suttie, J.W., McCormick, D.B., Machlin, L.J., Eds.; Marcel Dekker: New York, NY, USA, 2001. [Google Scholar]
- Nicolaou, K.C.; Chen, J.S.; Edmonds, D.J.; Estrada, A.A. Recent advances in the chemistry and biology of naturally occurring antibiotics. Angew. Chem. Int. Ed. 2009, 48, 660–719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lipshutz, B.H.; Mollard, P.; Pfeiffer, S.S.; Chrisman, W. A short, highly efficient synthesis of coenzyme Q10. J. Am. Chem. Soc. 2002, 124, 14282–14283. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, S.; Mascio, P.; Murphy, M.E.; Sies, H. Physical and chemical scavenging of singlet molecular oxygen by tocopherols. Arch. Biochem. Biophys. 1990, 277, 101–108. [Google Scholar] [CrossRef]
- Bonrath, W.; Eggersdorfer, M.; Netscher, T. Catalysis in the industrial preparation of vitamins and nutraceuticals. Catal. Today 2007, 121, 45–47. [Google Scholar] [CrossRef]
- Dehn, R.; Kraus, M.; Weis, M.; Danz, M.; Teles, J.H. Method for Producing 2,3,5-trimethyl benzoquinone by Oxidation of 2,3,6-trimethylphenol. Patent WO2015000767A1, 8 January 2015. [Google Scholar]
- Sun, H.; Harms, K.; Sundermeyer, J. Aerobic oxidation of 2,3,6-trimethylphenol to trimethyl-1,4-benzoquinone with copper(II) chloride as catalyst in ionic liquid and structure of the active species. J. Am. Chem. Soc. 2004, 126, 9550–9551. [Google Scholar] [CrossRef]
- Liangning Hu, M.S.; Yu, J.; Xin, H.; An, Z.; Sun, W. Aerobic water–based oxidation of 2,3,6-trimethylphenol to trimethyl-1,4-benzoquinone over copper(II) nitrate catalyst. Chem. Sel. 2017, 2, 949–952. [Google Scholar]
- Shimizu, M.; Orita, H.; Hayakawa, T.; Takehira, K. A convenient synthesis of alkyl-substituted p-benzoquinones from phenols by a H2O2/heteropolyacid system. Tetrahedron Lett. 1989, 30, 471–474. [Google Scholar] [CrossRef]
- Adam, W.; Herrmann, W.A.; Lin, J.; Saha-Moeller, C.R. Catalytic oxidation of phenols to p-quinones with the hydrogen peroxide and methyltrioxorhenium(VII) system. J. Org. Chem. 1994, 59, 8281–8283. [Google Scholar] [CrossRef]
- Wienhoefer, G.; Schröder, K.; Möller, K.; Junge, K.; Beller, M. A novel process for selective ruthenium-catalyzed oxidation of naphthalenes and phenols. Adv. Synth. Catal. 2010, 352, 1615–1620. [Google Scholar] [CrossRef]
- Möller, K.; Wienhöfer, G.; Schröder, K.; Join, B.; Junge, K.; Beller, M. Selective iron-catalyzed oxidation of phenols and arenes with hydrogen peroxide: Synthesis of vitamin e intermediates and vitamin K3. Chem. Eur. J. 2010, 16, 10300–10303. [Google Scholar] [CrossRef]
- Trubitsyna, T.A.; Kholdeeva, O.A. Kinetics and mechanism of the oxidation of 2,3,6-trimethylphenol with hydrogen peroxide in the presence of Ti-monosubstituted polyoxometalates. Kinet. Catal. 2008, 49, 371–378. [Google Scholar] [CrossRef]
- Kholdeeva, O.A.; Ivanchikova, I.D.; Guidotti, M.; Ravasio, N.; Sgobba, M.; Barmatova, M.V. How to reach 100% selectivity in H2O2-based oxidation of 2,3,6-trimethylphenol to trimethyl-p-benzoquinone over Ti,Si-catalysts. Catal. Today 2009, 141, 330–336. [Google Scholar] [CrossRef]
- Torbina, V.V.; Vodyankin, A.A.; Ivanchikova, I.D.; Kholdeeva, O.A.; Vodyankina, O.V. Support pretreatment effect on the catalytic properties and reusability of silica-supported titania catalysts in 2,3,6-trimethylphenol oxidation with hydrogen peroxide. Kinet. Catal. 2015, 56, 369–374. [Google Scholar] [CrossRef]
- Evtushok, V.Y.; Suboch, A.N.; Podyacheva, O.Y.; Stonkus, O.A.; Zaikovskii, V.I.; Chesalov, Y.A.; Kibis, L.S.; Kholdeeva, O.A. Highly efficient catalysts based on divanadium-substituted polyoxometalate and N-doped carbon nanotubes for selective oxidation of alkylphenols. ACS Catal. 2018, 8, 1297–1307. [Google Scholar] [CrossRef]
- Zalomaeva, O.V.; Evtushok, V.Y.; Maksimov, G.M.; Maksimovskaya, R.I.; Kholdeeva, O.A. Synthesis of coenzyme Q0 through divanadium-catalyzed oxidation of 3,4,5-trimethoxytoluene with hydrogen peroxide. Dalton Trans. 2017, 46, 5202–5209. [Google Scholar] [CrossRef] [Green Version]
- Ivanchikova, I.D.; Maksimchuk, N.V.; Maksimovskaya, R.I.; Maksimov, G.M.; Kholdeeva, O.A. Highly selective oxidation of alkylphenols to p-Benzoquinones with aqueous hydrogen peroxide catalyzed by divanadium-substituted polyoxotungstates. ACS Catal. 2014, 4, 2706–2713. [Google Scholar] [CrossRef]
- Latos, P.; Culkin, A.; Barteczko, N.; Boncel, S.; Jurczyk, S.; Brown, L.C.; Nockemann, P.; Chrobok, A.; Swadźba-Kwaśny, M. Water-tolerant trifloaluminate ionic liquids: New and unique lewis acidic catalysts for the synthesis of chromane. Front. Chem. 2018, 6, 535. [Google Scholar] [CrossRef]
- Latos, P.; Szelwicka, A.; Boncel, S.; Jurczyk, S.; Swadźba-Kwaśny, M.; Chrobok, A. Highly efficient synthesis of alkyl levulinates from alpha-angelica lactone, catalyzed with lewis acidic trifloaluminate ionic liquids supported on carbon nanotubes. ACS Sustain. Chem. Eng. 2019, 7, 5184–5191. [Google Scholar] [CrossRef]
- Estager, J.; Oliferenko, A.A.; Seddon, K.R.; Swadźba-Kwaśny, M. Chlorometallate(III) ionic liquids as Lewis acidic catalysts—A quantitative study of acceptor properties. Dalton Trans. 2010, 39, 11375–11382. [Google Scholar] [CrossRef]
- Zaikov, G.E.; Maizus, Z.K. Effect of solvents on rates and routes of oxidation reactions. Adv. Chem. 1968, 12, 150–164. [Google Scholar]
- Brown, L.C.; Hogg, J.M.; Swadźba-Kwaśny, M. Lewis Acidic Ionic Liquids. Top Curr. Chem. 2017, 375, 78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cinke, M.; Li, J.; Chen, B.; Cassell, A.; Delzeit, L.; Han, J.; Meyyappan, M. Pore structure of raw and purified HiPco single-walled carbon nanotubes. Chem. Phys. Lett. 2002, 365, 69–74. [Google Scholar] [CrossRef]
- Musso, S.; Porro, S.; Vinante, M.; Vanzetti, L.; Ploeger, R.; Giorcelli, M.; Possetti, B.; Trotta, F.; Pederzolli, C.; Tagliaferro, A. Modification of MWNTs obtained by thermal-CVD. Diam. Relat. Mater. 2007, 16, 1183–1187. [Google Scholar] [CrossRef]





| Entry | IL | mol% of IL 1 | TMP Conversion (%) | TMBQ Selectivity (%) |
|---|---|---|---|---|
| 1 | [emim][OTf]-Al(OTf)3, χAl(OTf)3 = 0.15 | 3 | 41 | 73 |
| 2 | [emim][OTf]-Al(OTf)3, χAl(OTf)3 = 0.25 | 3 | 77 | 79 |
| 3 | [emim][OTf]-Al(OTf)3, χAl(OTf)3 = 0.25 | 2 | 83 | 72 |
| Entry | Solvent | TMP Conversion (%) | TMBQ Selectivity (%) |
|---|---|---|---|
| 1 | CH3COOH | 88 | 92 |
| 2 1 | CH3COOH | 31 | 89 |
| 3 2 | CH3COOH | 79 | 79 |
| 4 | CH3COOH + CH3CN | 69 | 80 |
| 5 | CH3CN | 6 | 86 |
| 6 | n-BuOH | 10 | 73 |
| 7 | MeOH | 9 | 71 |
| Entry | CH3COOH (cm3) | Temperature (°C) | Time (h) | TMP 1 Conversion (%) | TMBQ 1 Selectivity (%) |
|---|---|---|---|---|---|
| 1 | 1 | 70 | 2 | 77 | 78 |
| 2 | 1 | 70 | 4 | 99 | 84 |
| 3 | 3 | 70 | 2 | 79 | 79 |
| 4 | 3 | 70 | 4 | 91 | 81 |
| 5 | 1 | 100 | 2 | 99 | 86 |
| Entry | Concentration of H2O2 (%) | Molar Ratio of TMP:H2O2 | TMP 1 Conversion (%) | TMBQ 1 Selectivity (%) |
|---|---|---|---|---|
| 1 | 35 | 1:3 | 87 | 77 |
| 2 | 60 | 1:3 | 95 | 81 |
| 3 | 60 | 1:2 | 77 | 82 |
| 4 | 60 | 1:4 | 99 | 84 |
| Entry | Catalyst | TMP 1 Conversion (%) | TMBQ 1 Selectivity (%) | TON 2 (10−3) | TOF 3 (min−1) |
|---|---|---|---|---|---|
| 1 | [emim][OTf]-Al(OTf)3, χAl(OTf)3 = 0.25 | 99 | 84 | 83.2 | 347 |
| 2 | MWCNT-[emim][OTf]-Al(OTf)3, χAl(OTf)3 = 0.25 | 98 | 84 | 79.8 | 333 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Latos, P.; Siewniak, A.; Barteczko, N.; Jurczyk, S.; Boncel, S.; Chrobok, A. Highly Active Trifloaluminate Ionic Liquids as Recyclable Catalysts for Green Oxidation of 2,3,6-Trimethylphenol to Trimethyl-1,4-Benzoquinone. Catalysts 2020, 10, 1469. https://doi.org/10.3390/catal10121469
Latos P, Siewniak A, Barteczko N, Jurczyk S, Boncel S, Chrobok A. Highly Active Trifloaluminate Ionic Liquids as Recyclable Catalysts for Green Oxidation of 2,3,6-Trimethylphenol to Trimethyl-1,4-Benzoquinone. Catalysts. 2020; 10(12):1469. https://doi.org/10.3390/catal10121469
Chicago/Turabian StyleLatos, Piotr, Agnieszka Siewniak, Natalia Barteczko, Sebastian Jurczyk, Sławomir Boncel, and Anna Chrobok. 2020. "Highly Active Trifloaluminate Ionic Liquids as Recyclable Catalysts for Green Oxidation of 2,3,6-Trimethylphenol to Trimethyl-1,4-Benzoquinone" Catalysts 10, no. 12: 1469. https://doi.org/10.3390/catal10121469