Accelerating the Oxygen Reduction Reaction and Oxygen Evolution Reaction Activities of N and P Co-Doped Porous Activated Carbon for Li-O2 Batteries
Abstract
:1. Introduction
2. Results and Discussion
2.1. Preparation and Morphological Properties
2.2. Structral and Chemical Properties
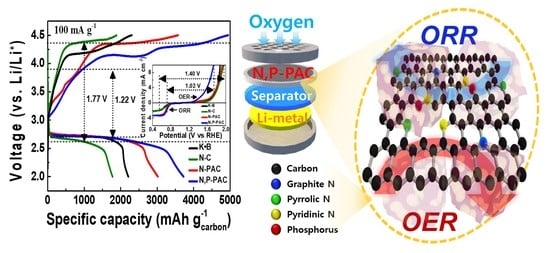
2.3. Electrochemical Catalytic Properties
3. Materials and Methods
3.1. Experimental Details
3.2. Characterization
3.2.1. Structures and Morphologies
3.2.2. Electrochemical Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Girishkumar, G.; McCloskey, B.; Luntz, A.C.; Swanson, S.; Wilcke, W. Lithium-Air Battery: Promise and Challenges. J. Phys. Chem. Lett. 2010, 1, 2193–2203. [Google Scholar] [CrossRef]
- Geng, D.; Ding, N.; Hor, T.S.A.; Chien, S.W.; Liu, Z.; Wuu, D.; Sun, X.; Zong, Y. From Lithium-Oxygen to Lithium-Air Batteries: Challenges and Opportunities. Adv. Energy Mater. 2016, 6, 1502164–1502177. [Google Scholar] [CrossRef]
- Balaish, M.; Kraytsberg, A.; Ein-Eli, Y. A critical review on lithium–air battery electrolytes. Phys. Chem. Chem. Phys. 2014, 16, 2801–2822. [Google Scholar] [CrossRef] [PubMed]
- Aurbach, D.; McCloskey, B.D.; Nazar, L.F.; Bruce, P.G. Advances in understanding mechanisms underpinning lithium–air batteries. Nat. Energy 2016, 1, 16128–16138. [Google Scholar] [CrossRef]
- Lu, Y.-C.; Shao-Horn, Y. Probing the Reaction Kinetics of the Charge Reactions of Nonaqueous Li−O2 Batteries. J. Phys. Chem. Lett. 2013, 4, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Fan, W.; Guo, X.; Meng, F.; Liu, X. Positive Role of Surface Defects on Carbon Nanotube Cathodes in Overpotential and Capacity Retention of Rechargeable Lithium−Oxygen Batteries. ACS Appl. Mater. Interfaces 2014, 6, 21567–21575. [Google Scholar] [CrossRef] [PubMed]
- Shui, J.; Lin, Y.; Connell, J.W.; Xu, J.; Fan, X.; Dai, L. Nitrogen-Doped Holey Graphene for High-Performance Rechargeable Li−O2 Batteries. ACS Energy Lett. 2016, 1, 260–265. [Google Scholar] [CrossRef]
- Ma, Z.; Yuan, X.; Li, L.; Ma, Z.-F.; Wilkinson, D.P.; Zhang, L.; Zhang, J. A review of cathode materials and structures for rechargeable lithium–air batteries. Energy Environ. Sci. 2015, 8, 2144–2198. [Google Scholar] [CrossRef]
- Wen, Z.; Shen, C.; Lu, Y. Air Electrode for the Lithium–Air Batteries: Materials and Structure Designs. ChemSusChem 2015, 80, 270–287. [Google Scholar]
- Han, S.J.; Ameen, M.; Hanifah, M.F.R.; Aqsha, A.; Bilad, M.R.; Jaafar, J.; Kheawhom, S. Catalytic Evaluation of Nanoflower Structured Manganese Oxide Electrocatalyst for Oxygen Reduction in Alkaline Media. Catalysts 2020, 10, 822–835. [Google Scholar]
- Liu, X.; Zhou, Y.; Zhou, W.; Li, L.; Huang, S.; Chen, S. Biomass-derived nitrogen self-doped porous carbon as effective metal-free catalysts for oxygen reduction reaction. Nanoscale 2015, 7, 6136–6142. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Yu, J.; Houston, J.; Flores, N.; Luo, H. Biomass derived porous nitrogen doped carbon for electrochemical devices. Green Energy Environ. 2017, 2, 84–99. [Google Scholar] [CrossRef]
- Zhang, G.; Yao, Y.; Zhao, T.; Wang, M.; Chen, R. From Black Liquor to Green Energy Resource: Positive Electrode Materials for Li−O2 Battery with High Capacity and Long Cycle Life. ACS Appl. Mater. Interfaces 2020, 12, 16521–16530. [Google Scholar] [CrossRef] [PubMed]
- Zhou, N.; Wang, N.; Wu, Z.; Li, L. Probing Active Sites on Metal-Free, Nitrogen-Doped Carbons for Oxygen Electroreduction: A Review. Catalysts 2018, 8, 509. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Ma, J.; Yang, H.; Lu, G.; Yang, S.; Chang, Z. Nitrogen and Cobalt Co-Coped Carbon Materials Derived from Biomass Chitin as High-Performance Electrocatalyst for Aluminum-Air Batteries. Catalysts 2020, 9, 954. [Google Scholar] [CrossRef] [Green Version]
- An, G.-H.; Lee, D.-Y.; Ahn, H.-J. Tofu-derived carbon framework with embedded ultrasmall tin nanocrystals for high-performance energy storage devices. J. Alloy. Compd. 2017, 722, 60–68. [Google Scholar] [CrossRef]
- An, G.-H.; Kim, H.; Ahn, H.-J. Surface functionalization of nitrogen-doped carbon derived from protein as anode material for lithium storage. Appl. Surf. Sci. 2019, 463, 18–26. [Google Scholar] [CrossRef]
- Wang, J.; Kaskel, S. KOH activation of carbon-based materials for energy storage. J. Mater. Chem. 2012, 22, 23710–23725. [Google Scholar] [CrossRef]
- Wu, J.; Yang, Z.; Sun, Q.; Li, X.; Strasser, P.; Yang, R. Synthesis and electrocatalytic activity of phosphorus-doped carbon xerogel for oxygen reduction. Electrochim. Acta 2014, 127, 53–60. [Google Scholar] [CrossRef]
- Jiang, Z.-L.; Sun, H.; Shi, W.-K.; Cheng, J.-Y.; Hu, J.-Y.; Guo, H.-L.; Gao, M.-Y.; Zhou, H.; Sun, S.-G. P-Doped Hive-like Carbon Derived from Pinecone Biomass as Efficient Catalyst for Li−O2 Battery. ACS Sustainable Chem. Eng. 2019, 7, 14161–14169. [Google Scholar] [CrossRef]
- Zhao, Z.; Xia, Z. Design Principles for Dual-Element-Doped Carbon Nanomaterials as Efficient Bifunctional Catalysts for Oxygen Reduction and Evolution Reactions. ACS Catal. 2016, 6, 1553–1558. [Google Scholar] [CrossRef]
- Kim, K.-H.; Shin, D.-Y.; Ahn, H.-J. Ecklonia cava based mesoporous activated carbon for high-rate energy storage devices. J. Ind. Eng. Chem. 2020, 84, 393–399. [Google Scholar] [CrossRef]
- Shin, D.-Y.; Jo, H.-G.; Ahn, H.-J. Accelerating lithium storage capability of cobalt sulfide encapsulated within anion dual-doped mesoporous carbon nanofibers. Appl. Surf. Sci. 2020, 527, 146895–146903. [Google Scholar] [CrossRef]
- Shin, D.-Y.; Sung, K.-W.; Ahn, H.-J. Synergistic effect of heteroatom-doped activated carbon for ultrafast charge storage kinetics. Appl. Surf. Sci. 2019, 478, 499–504. [Google Scholar] [CrossRef]
- Li, S.; Wang, M.; Yao, Y.; Zhao, T.; Yang, L.; Wu, F. Effect of the Activation Process on the Microstructure and Electrochemical Properties of N-Doped Carbon Cathodes in Li−O2 Batteries. ACS Appl. Mater. Interfaces 2019, 11, 34997–35004. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.; Lin, Y.; Fan, J.; Li, Q.; Shi, P.; Xu, Q.; Min, Y. Ternary N, S, and P-Doped Hollow Carbon Spheres Derived from Polyphosphazene as Pd Supports for Ethanol Oxidation Reaction. Catalysts 2019, 9, 114. [Google Scholar] [CrossRef] [Green Version]
- Li, P.; Hwang, J.-Y.; Park, S.-M.; Sun, Y.-K. Superior lithium/potassium storage capability of nitrogen-rich porous carbon nanosheets derived from petroleum coke. J. Mater. Chem. A 2018, 6, 12551–12558. [Google Scholar] [CrossRef]
- Hou, J.; Cao, C.; Idress, F.; Ma, X. Hierarchical Porous Nitrogen-Doped Carbon Nanosheets Derived from Silk for Ultrahigh-Capacity Battery Anodes and Supercapacitors. ACS Nano 2015, 9, 2556–2564. [Google Scholar] [CrossRef]
- An, G.-H.; Jo, H.-G.; Ahn, H.-J. Surface effect of platinum catalyst-decorated mesoporous carbon support using the dissolution of zinc oxide for methanol oxidation. Appl. Surf. Sci. 2019, 473, 511–515. [Google Scholar] [CrossRef]
- Xie, Y.; Yang, Y.; Zhou, Q.; Wang, L.; Yao, Y.; Zhu, X.; Fang, L.; Zhao, H.; Zhang, J. Metal-Free N, P-Codoped Porous Carbon Fibers for Oxygen Reduction Reactions. J. Electrochem. Soc. 2019, 166, 549–555. [Google Scholar] [CrossRef]
- Jiang, H.; Zhu, Y.; Feng, Q.; Su, Y.; Yang, X.; Li, C. Nitrogen and Phosphorus Dual-Doped Hierarchical Porous Carbon Foams as Efficient Metal-Free Electrocatalysts for Oxygen Reduction Reactions. Chem. Eur. J. 2014, 20, 3106–3112. [Google Scholar] [CrossRef] [PubMed]
- Lei, W.; Deng, Y.-P.; Li, G.; Cano, Z.P.; Wang, X.; Luo, D.; Liu, Y.; Wang, D.; Chen, Z. Two-Dimensional Phosphorus-Doped Carbon Nanosheets with Tunable Porosity for Oxygen Reactions in Zinc-Air Batteries. ACS Catal. 2018, 8, 2464–2472. [Google Scholar] [CrossRef]
- Li, J.-C.; Hou, P.-X.; Cheng, M.; Liu, C.; Cheng, H.-M.; Shao, M. Carbon nanotube encapsulated in nitrogen and phosphorus co-doped carbon as a bifunctional electrocatalyst for oxygen reduction and evolution reactions. Carbon 2018, 139, 156–163. [Google Scholar] [CrossRef]
- Yao, Z.; Fan, R.; Ji, W.; Yan, T.; Hu, M. N8- Polynitrogen Stabilized on Nitrogen-Doped Carbon Nanotubes as an Efficient Electrocatalyst for Oxygen Reduction Reaction. Catalysts 2020, 10, 864. [Google Scholar] [CrossRef]
- Kim, J.-H.; Kannan, A.G.; Woo, H.-S.; Jin, D.-G.; Kim, W.; Ryu, K.; Kim, D.-W. A bi-functional metal-free catalyst composed of dual-doped graphene and mesoporous carbon for rechargeable lithium–oxygen batteries. J. Mater. Chem. A 2015, 3, 18456–18465. [Google Scholar] [CrossRef]
- Balaish, M.; Jung, J.-W.; Kim, I.-D.; E-Eli, Y. A Critical Review on Functionalization of Air-Cathodes for Nonaqueous Li–O2 Batteries. Adv. Funct. Mater. 2020, 30, 1808303–1808336. [Google Scholar] [CrossRef]
- Gallant, B.M.; Mitchell, R.R.; Kwabi, D.G.; Zhou, J.; Zuin, L.; Thompson, C.V.; Shao-Horn, Y. Chemical and Morphological Changes of Li−O2 Battery Electrodes upon Cycling. J. Phys. Chem. C. 2012, 116, 20800–20805. [Google Scholar] [CrossRef]
- Gao, K.; Wang, B.; Tao, L.; Cunning, B.V.; Zhang, Z.; Wang, S.; Ruoff, R.S.; Qu, L. Efficient Metal-Free Electrocatalysts from N-Doped Carbon Nanomaterials: Mono-Doping and Co-Doping. Adv. Mater. 2019, 31, 1805121–1805131. [Google Scholar] [CrossRef]
- Zhao, Z.; Gao, C.; Mac, K.; Lu, Y. Pyrolysis derived helically nitrogen-doped carbon nanotubes with uniform cobalt for high performance oxygen reduction. Appl. Surf. Sci. 2020, 504, 144380–144385. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, J.; Wu, Z.; Wang, S.; Wu, Y.; Liu, X. Heteroatom (Nitrogen/Sulfur)-Doped Graphene as an Efficient Electrocatalyst for Oxygen Reduction and Evolution Reactions. Catalysts 2018, 8, 475. [Google Scholar] [CrossRef] [Green Version]
- Nunes, M.; Rocha, I.M.; Fernandes, D.M.; Mestre, A.S.; Moura, C.N.; Carvalho, A.P.; Pereira, M.F.R.; Freire, C. Sucrose-derived activated carbons: Electron transfer properties and application as oxygen reduction electrocatalysts. RSC Adv. 2015, 5, 102919–102931. [Google Scholar] [CrossRef]
- An, G.-H.; Lee, Y.-G.; Ahn, H.-J. Multi-active sites of iron carbide nanoparticles on nitrogen@cobalt-doped carbon for a highly efficient oxygen reduction reaction. J. Alloy. Compd. 2018, 746, 177–184. [Google Scholar] [CrossRef]
- Lee, Y.-G.; Ahn, H.-J. Tri(Fe/N/F)-doped mesoporous carbons as efficient electrocatalysts for the oxygen reduction reaction. Appl. Surf. Sci. 2019, 487, 389–397. [Google Scholar] [CrossRef]
- Sun, J.; Wang, S.; Wang, Y.; Li, H.; Zhou, H.; Chen, B.; Zhang, X.; Chen, H.; Qu, K.; Zhao, J. One Simple Strategy towards Nitrogen and Oxygen Codoped Carbon Nanotube for Efficient Electrocatalytic Oxygen Reduction and Evolution. Catalysts 2019, 9, 159. [Google Scholar] [CrossRef] [Green Version]
- Marsudi, M.A.; Ma, Y.; Prakoso, B.; Hutani, J.J.; Wibowo, A.; Zong, Y.; Liu, Z.; Sumboja, A. Manganese Oxide Nanorods Decorated Table Sugar Derived Carbon as Efficient Bifunctional Catalyst in Rechargeable Zn-Air Batteries. Catalysts 2020, 10, 64. [Google Scholar] [CrossRef] [Green Version]
- Ryu, W.-H.; Yoon, T.-H.; Song, S.H.; Jeon, S.; Park, Y.-J.; Kim, I.-D. Bifunctional Composite Catalysts Using Co3O4 Nanofibers Immobilized on Nonoxidized Graphene Nanoflakes for High-Capacity and Long-Cycle Li−O2 Batteries. Nano Lett. 2013, 13, 4190–4197. [Google Scholar] [CrossRef]
- Xie, L.; Zhao, D.; Dai, J.; Wu, Z.; Li, L. Solvothermally Doping NiS2 Nanoparticles on Carbon with Ferric Ions for Efficient Oxygen Evolution Catalysis. Catalysts 2019, 9, 458. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Chen, C.; Cheng, Q.; Zou, L.; Zou, Z.; Yang, H. Binary Nitrogen Precursor-Derived Porous Fe-N-S/C Catalyst for Efficient Oxygen Reduction Reaction in a Zn-Air Battery. Catalysts 2018, 8, 158. [Google Scholar] [CrossRef] [Green Version]







| Samples | SBET (m2 g−1) | Total Pore Volume (p/p0 = 0.990) (cm3 g−1) | Average Pore Diameter (nm) | Pore Size Distribution | |
|---|---|---|---|---|---|
| Vmicro (%) | Vmeso (%) | ||||
| N-C | 117 | 0.19 | 6.53 | - | - |
| N-PAC | 2086 | 1.22 | 2.34 | 60% | 40% |
| N,P-PAC | 2473 | 1.53 | 2.48 | 55% | 45% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jo, H.-G.; Ahn, H.-J. Accelerating the Oxygen Reduction Reaction and Oxygen Evolution Reaction Activities of N and P Co-Doped Porous Activated Carbon for Li-O2 Batteries. Catalysts 2020, 10, 1316. https://doi.org/10.3390/catal10111316
Jo H-G, Ahn H-J. Accelerating the Oxygen Reduction Reaction and Oxygen Evolution Reaction Activities of N and P Co-Doped Porous Activated Carbon for Li-O2 Batteries. Catalysts. 2020; 10(11):1316. https://doi.org/10.3390/catal10111316
Chicago/Turabian StyleJo, Hyun-Gi, and Hyo-Jin Ahn. 2020. "Accelerating the Oxygen Reduction Reaction and Oxygen Evolution Reaction Activities of N and P Co-Doped Porous Activated Carbon for Li-O2 Batteries" Catalysts 10, no. 11: 1316. https://doi.org/10.3390/catal10111316