Metal-Based Electrocatalysts for High-Performance Lithium-Sulfur Batteries: A Review
Abstract
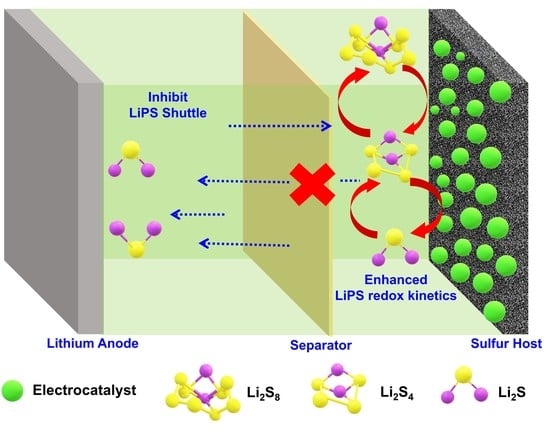
:1. Introduction
2. Electrocatalysis of Intermediate LiPS
3. Metals-Sulfide Interactions in the Electrocatalysis of LiPS Redox Reaction
4. Metal Oxide-Sulfide Interactions in Electrocatalysis of LiPS Redox Reactions
5. Metal Sulfides-Sulfide Interactions in Electrocatalysis of LiPS Redox Reactions
6. Metal Carbide-Sulfide Interactions in Electrocatalysis of LiPS Redox Reactions
7. Summary and Outlook
- (i)
- As the intermediate LiPS forms with different chain lengths during the reactions and undergoes manifold (electro) chemical transformations, their binding strength varies from surface to surface. In such circumstances, the cathode surfaces no longer can offer a ubiquitous anchoring effect towards all the intermediate LiPS, and subsequently, some tend to undergo dissolution. Exploring the design principle for anchoring of LiPS on the cathode substrates that are capable of adsorbing all the intermediate LiPS while catalyzing the subsequent redox reactions is imperative to completely restrain the PS shuttle.
- (ii)
- The catalysts with high surface area and exceptional electronic conductivity need to be developed to promote Li+ transportation in the inner parts of cathodes to access the active materials while facilitating the redox conversion reaction. This could provide an opportunity to achieve high sulfur loading with low electrolyte/sulfur ratio to realize high energy density Li-S batteries. Besides, maximizing the sulfur loading without compromising on the electrocatalytic activity holds the key to realize the effective utilization of the electrocatalysts and, ultimately, high energy density Li-S batteries. Additionally, catalytic cathodes should have a rigid structure with enough porosity to enable uniform distribution of active materials and accommodate volume changes during charge/discharge.
- (iii)
- An in-depth understanding of the phenomenon occurring at the electrode/electrolyte interface with theoretical and sophisticated in situ measurements is vital to understand the interactions of LiPS with the catalytic cathodes in real-time. This could reveal essential information such as the nature of such interactions, LiPS reaction pathways on catalyst cathodes during the entire reactions, etc., which are essential to elucidate the accelerated reversible redox pathways of sulfur redox reactions.
Author Contributions
Funding
Conflicts of Interest
References
- Bruce, P.G.; Freunberger, S.A.; Hardwick, L.J.; Tarascon, J.M. Li–O2 and Li–S batteries with high energy storage. Nat. Mater. 2012, 11, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Armand, M.; Tarascon, J.M. Building better batteries. Nature 2008, 451, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Miroshnikov, M.; Kato, K.; Babu, G.; Kumar, N.; Mahankali, K.; Hohenstein, E.; Wang, H.; Satapathy, S.; Divya, K.P.; Asare, H.; et al. Nature-Derived Sodium-Ion Battery: Mechanistic Insights into Na-Ion Coordination within Sustainable Molecular Cathode Materials. ACS Appl. Energy Mater. 2019, 2, 8596–8604. [Google Scholar] [CrossRef]
- Miroshnikov, M.; Mahankali, K.; Thangavel, N.K.; Satapathy, S.; Arava, L.M.R.; Ajayan, P.M.; John, G. Bioderived Molecular Electrodes for Next-Generation Energy-Storage Materials. ChemSusChem 2020, 13, 2186–2204. [Google Scholar] [CrossRef]
- Manthiram, A.; Fu, Y.; Chung, S.H.; Zu, C.; Su, Y.S. Rechargeable lithium-sulfur batteries. Chem. Rev. 2014, 114, 11751–11787. [Google Scholar] [CrossRef]
- Li, Z.; Huang, Y.M.; Yuan, L.X.; Hao, Z.X.; Huang, Y.H. Status and prospects in sulfur-carbon composites as cathode materials for rechargeable lithium-sulfur batteries. Carbon 2015, 92, 41–63. [Google Scholar] [CrossRef]
- Barchasz, C.; Leprêtre, J.C.; Alloin, F.; Patoux, S. New insights into the limiting parameters of the Li/S rechargeable cell. J. Power Sources 2012, 199, 322–330. [Google Scholar] [CrossRef]
- Jung, Y.; Kim, S. New approaches to improve cycle life characteristics of lithium–sulfur cells. Electrochem. Commun. 2007, 9, 249–254. [Google Scholar] [CrossRef]
- Barghamadi, M.; Kapoor, A.; Wen, C. A review on Li-S batteries as a high efficiency rechargeable lithium battery. J. Electrochem. Soc. 2013, 160, A1256–A1263. [Google Scholar] [CrossRef]
- Ji, X.; Lee, K.T.; Nazar, L.F. A highly ordered nanostructured carbon-sulphur cathode for lithium-sulphur batteries. Nat. Mater. 2009, 8, 500–506. [Google Scholar] [CrossRef]
- Angulakshmi, N.; Stephan, A.M. Efficient electrolytes for lithium–sulfur batteries. Front. Energy Res. 2015, 3, 17. [Google Scholar] [CrossRef] [Green Version]
- Mikhaylik, Y.V.; Akridge, J.R. Polysulfide shuttle study in the Li/S battery system. J. Electrochem. Soc. 2004, 151, A1969–A1976. [Google Scholar] [CrossRef]
- Mahankali, K.; Thangavel, N.K.; Gopchenko, D.; Arava, L.M.R. Atomically Engineered Transition Metal Dichalcogenides for Liquid Polysulfide Adsorption and Their Effective Conversion in Li-S Batteries. ACS Appl. Mater. Interfaces 2020, 12, 27112–27121. [Google Scholar] [CrossRef] [PubMed]
- Cheon, S.E.; Ko, K.S.; Cho, J.H.; Kim, S.W.; Chin, E.Y.; Kim, H.T. Rechargeable lithium sulfur battery I. Structural change of sulfur cathode during discharge and charge. J. Electrochem. Soc. 2003, 150, A796–A799. [Google Scholar] [CrossRef]
- Mahankali, K.; Thangavel, N.K.; Reddy Arava, L.M. In Situ Electrochemical Mapping of Lithium–Sulfur Battery Interfaces Using AFM–SECM. Nano Lett. 2019, 19, 5229–5236. [Google Scholar] [CrossRef]
- Luo, Y.; Guo, L.; Xiao, M.; Wang, S.; Ren, S.; Han, D.; Meng, Y. Strategies for inhibiting anode dendrite growth in lithium–sulfur batteries. J. Mater. Chem. A 2020, 8, 4629–4646. [Google Scholar] [CrossRef]
- Xu, X.; Wang, S.; Wang, H.; Xu, B.; Hu, C.; Jin, Y.; Liu, J.; Yan, H. The suppression of lithium dendrite growth in lithium sulfur batteries: A review. J. Energy Storage 2017, 13, 387–400. [Google Scholar] [CrossRef]
- Xie, K.; Yuan, K.; Zhang, K.; Shen, C.; Lv, W.; Liu, X.; Wang, J.G.; Wei, B. Dual Functionalities of Carbon Nanotube Films for Dendrite-Free and High Energy-High Power Lithium-Sulfur Batteries. ACS Appl. Mater. Interfaces 2017, 9, 4605–4613. [Google Scholar] [CrossRef]
- Chen, L.; Shaw, L.L. Recent advances in lithium–sulfur batteries. J. Power Sources 2014, 267, 770–783. [Google Scholar] [CrossRef]
- Choi, Y.J.; Chung, Y.D.; Baek, C.Y.; Kim, K.W.; Ahn, H.J.; Ahn, J.H. Effects of carbon coating on the electrochemical properties of sulfur cathode for lithium/sulfur cell. J. Power Sources 2008, 184, 548–552. [Google Scholar] [CrossRef]
- Zhang, B.; Lai, C.; Zhou, Z.; Gao, X. Preparation and electrochemical properties of sulfur–acetylene black composites as cathode materials. Electrochim. Acta 2009, 54, 3708–3713. [Google Scholar] [CrossRef]
- Liang, C.; Dudney, N.J.; Howe, J.Y. Hierarchically structured sulfur/carbon nanocomposite material for high-energy lithium battery. Chem. Mater. 2009, 21, 4724–4730. [Google Scholar] [CrossRef]
- Guo, J.; Xu, Y.; Wang, C. Sulfur-impregnated disordered carbon nanotubes cathode for lithium-sulfur batteries. Nano Lett. 2011, 11, 4288–4294. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Yuan, H.; Qiu, X.; Chen, L.; Zhu, W. Improvement of cycle property of sulfur-coated multi-walled carbon nanotubes composite cathode for lithium/sulfur batteries. J. Power Sources 2009, 189, 1141–1146. [Google Scholar] [CrossRef]
- He, G.; Evers, S.; Liang, X.; Cuisinier, M.; Garsuch, A.; Nazar, L.F. Tailoring porosity in carbon nanospheres for lithium-sulfur battery cathodes. ACS Nano 2013, 7, 10920–10930. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.; Rao, M.; Zheng, H.; Zhang, L.; Li, Y.; Duan, W.; Guo, J.; Cairns, E.J.; Zhang, Y. Graphene oxide as a sulfur immobilizer in high performance lithium/sulfur cells. J. Am. Chem. Soc. 2011, 133, 18522–18525. [Google Scholar] [CrossRef]
- Babu, G.; Arava, L.M.R. Graphene-decorated graphite–sulfur composite as a high-tap-density electrode for Li–S batteries. RSC Adv. 2015, 5, 47621–47627. [Google Scholar] [CrossRef]
- Park, M.S.; Yu, J.S.; Kim, K.J.; Jeong, G.; Kim, J.H.; Yim, T.; Jo, Y.N.; Hwang, U.; Kang, S.; Woo, T. Porous carbon spheres as a functional conducting framework for use in lithium–sulfur batteries. RSC Adv. 2013, 3, 11774–11781. [Google Scholar] [CrossRef]
- Liu, X.; Huang, J.Q.; Zhang, Q.; Mai, L. Nanostructured Metal Oxides and Sulfides for Lithium-Sulfur Batteries. Adv. Mater. 2017, 29, 1601759. [Google Scholar] [CrossRef]
- He, J.; Manthiram, A. A review on the status and challenges of electrocatalysts in lithium-sulfur batteries. Energy Storage Mater. 2019, 20, 55–70. [Google Scholar] [CrossRef]
- Song, M.S.; Han, S.C.; Kim, H.S.; Kim, J.H.; Kim, K.T.; Kang, Y.M.; Ahn, H.J.; Dou, S.; Lee, J.Y. Effects of nanosized adsorbing material on electrochemical properties of sulfur cathodes for Li/S secondary batteries. J. Electrochem. Soc. 2004, 151, A791–A795. [Google Scholar] [CrossRef]
- Choi, Y.; Jung, B.; Lee, D.; Jeong, J.; Kim, K.; Ahn, H.; Cho, K.; Gu, H. Electrochemical properties of sulfur electrode containing nano Al2O3 for lithium/sulfur cell. Phys. Scr. 2007, 2007, 62. [Google Scholar] [CrossRef]
- Pang, Q.; Kundu, D.; Cuisinier, M.; Nazar, L.F. Surface-enhanced redox chemistry of polysulphides on a metallic and polar host for lithium-sulphur batteries. Nat. Commun. 2014, 5, 4759. [Google Scholar] [CrossRef] [PubMed]
- Ponraj, R.; Kannan, A.G.; Ahn, J.H.; Kim, D.-W. Improvement of cycling performance of lithium–sulfur batteries by using magnesium oxide as a functional additive for trapping lithium polysulfide. ACS Appl. Mater. Interfaces 2016, 8, 4000–4006. [Google Scholar] [CrossRef]
- Cao, B.; Li, D.; Hou, B.; Mo, Y.; Yin, L.; Chen, Y. Synthesis of double-shell SnO2@ C hollow nanospheres as sulfur/sulfide cages for lithium–sulfur batteries. ACS Appl. Mater. Interfaces 2016, 8, 27795–27802. [Google Scholar] [CrossRef]
- Kong, Y.; Luo, J.; Jin, C.; Yuan, H.; Sheng, O.; Zhang, L.; Fang, C.; Zhang, W.; Huang, H.; Xia, Y. Enhanced sulfide chemisorption by conductive Al-doped ZnO decorated carbon nanoflakes for advanced Li–S batteries. Nano Res. 2018, 11, 477–489. [Google Scholar] [CrossRef]
- Rehman, S.; Guo, S.; Hou, Y. Rational Design of Si/SiO2@ Hierarchical Porous Carbon Spheres as Efficient Polysulfide Reservoirs for High-Performance Li–S Battery. Adv. Mater. 2016, 28, 3167–3172. [Google Scholar] [CrossRef]
- Liang, Z.; Zheng, G.; Li, W.; Seh, Z.W.; Yao, H.; Yan, K.; Kong, D.; Cui, Y. Sulfur cathodes with hydrogen reduced titanium dioxide inverse opal structure. ACS Nano 2014, 8, 5249–5256. [Google Scholar] [CrossRef]
- Cao, J.; Chen, C.; Zhao, Q.; Zhang, N.; Lu, Q.; Wang, X.; Niu, Z.; Chen, J. A Flexible Nanostructured Paper of a Reduced Graphene Oxide-Sulfur Composite for High-Performance Lithium-Sulfur Batteries with Unconventional Configurations. Adv. Mater. 2016, 28, 9629–9636. [Google Scholar] [CrossRef]
- Ma, G.; Wen, Z.; Jin, J.; Lu, Y.; Wu, X.; Wu, M.; Chen, C. Hollow polyaniline sphere@ sulfur composites for prolonged cycling stability of lithium–sulfur batteries. J. Mater. Chem. A 2014, 2, 10350–10354. [Google Scholar] [CrossRef]
- Ma, G.; Wen, Z.; Jin, J.; Lu, Y.; Wu, X.; Liu, C.; Chen, C. Enhancement of long stability of Li–S battery by thin wall hollow spherical structured polypyrrole based sulfur cathode. RSC Adv. 2014, 4, 21612–21618. [Google Scholar] [CrossRef]
- Yang, T.; Wang, X.; Wang, D.; Li, S.; Xie, D.; Zhang, X.; Xia, X.; Tu, J. Facile and scalable synthesis of nanosized core–shell Li2S@ C composite for high-performance lithium–sulfur batteries. J. Mater. Chem. A 2016, 4, 16653–16660. [Google Scholar] [CrossRef]
- Yu, R.; Chung, S.-H.; Chen, C.-H.; Manthiram, A. A core–shell cathode substrate for developing high-loading, high-performance lithium–sulfur batteries. J. Mater. Chem. A 2018, 6, 24841–24847. [Google Scholar] [CrossRef]
- Li, L.; Hou, L.; Cheng, J.; Simmons, T.; Zhang, F.; Zhang, L.T.; Linhardt, R.J.; Koratkar, N. A flexible carbon/sulfur-cellulose core-shell structure for advanced lithium–sulfur batteries. Energy Storage Mater. 2018, 15, 388–395. [Google Scholar] [CrossRef]
- Bucur, C.B.; Muldoon, J.; Lita, A. A layer-by-layer supramolecular structure for a sulfur cathode. Energy Environ. Sci. 2016, 9, 992–998. [Google Scholar] [CrossRef]
- Seh, Z.W.; Li, W.; Cha, J.J.; Zheng, G.; Yang, Y.; McDowell, M.T.; Hsu, P.C.; Cui, Y. Sulphur–TiO2 yolk–shell nanoarchitecture with internal void space for long-cycle lithium–sulphur batteries. Nat. Commun. 2013, 4, 1–6. [Google Scholar]
- Zhou, W.; Yu, Y.; Chen, H.; DiSalvo, F.J.; Abruna, H.D. Yolk-shell structure of polyaniline-coated sulfur for lithium-sulfur batteries. J. Am. Chem. Soc. 2013, 135, 16736–16743. [Google Scholar] [CrossRef] [PubMed]
- Pei, F.; Lin, L.; Ou, D.; Zheng, Z.; Mo, S.; Fang, X.; Zheng, N. Self-supporting sulfur cathodes enabled by two-dimensional carbon yolk-shell nanosheets for high-energy-density lithium-sulfur batteries. Nat. Commun. 2017, 8, 482. [Google Scholar] [CrossRef] [Green Version]
- Liang, X.; Nazar, L.F. In Situ Reactive Assembly of Scalable Core-Shell Sulfur-MnO2 Composite Cathodes. ACS Nano 2016, 10, 4192–4198. [Google Scholar] [CrossRef]
- Evers, S.; Yim, T.; Nazar, L.F. Understanding the nature of absorption/adsorption in nanoporous polysulfide sorbents for the Li–S battery. J. Phys. Chem. C 2012, 116, 19653–19658. [Google Scholar] [CrossRef]
- Gopalakrishnan, D.; Lee, A.; Thangavel, N.K.; Arava, L.M.R. Facile synthesis of electrocatalytically active NbS 2 nanoflakes for an enhanced hydrogen evolution reaction (HER). Sustain. Energy Fuels 2018, 2, 96–102. [Google Scholar] [CrossRef]
- Kumar, T.N.; Chandrasekaran, N.; Phani, K.L. Structural and electronic modification of MoS2 nanosheets using S-doped carbon for efficient electrocatalysis of the hydrogen evolution reaction. Chem. Commun. 2015, 51, 5052–5055. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Gao, Y.; Hu, K.; Blanchard, P.Y.; Noël, J.M.; Nareshkumar, T.; Phani, K.L.; Friedman, G.; Gogotsi, Y.; Mirkin, M.V. Electrochemistry and electrocatalysis at single gold nanoparticles attached to carbon nanoelectrodes. ChemElectroChem 2015, 2, 58–63. [Google Scholar] [CrossRef]
- Kumar, T.N.; Sivabalan, S.; Chandrasekaran, N.; Phani, K.L. Synergism between polyurethane and polydopamine in the synthesis of Ni–Fe alloy monoliths. Chem. Commun. 2015, 51, 1922–1925. [Google Scholar] [CrossRef] [PubMed]
- Stephens, I.E.; Ducati, C.; Fray, D.J. Correlating microstructure and activity for polysulfide reduction and oxidation at WS2 electrocatalysts. J. Electrochem. Soc. 2013, 160, A757–A768. [Google Scholar] [CrossRef] [Green Version]
- Munaiah, Y.; Suresh, S.; Dheenadayalan, S.; Pillai, V.K.; Ragupathy, P. Comparative Electrocatalytic performance of single-walled and multiwalled carbon nanotubes for zinc bromine redox flow batteries. J. Phys. Chem. C 2014, 118, 14795–14804. [Google Scholar] [CrossRef]
- Munaiah, Y.; Dheenadayalan, S.; Ragupathy, P.; Pillai, V.K. High performance carbon nanotube based electrodes for zinc bromine redox flow batteries. ECS J. Solid State Sci. Technol. 2013, 2, M3182. [Google Scholar] [CrossRef]
- Munaiah, Y.; Ragupathy, P.; Pillai, V.K. Single-step synthesis of halogenated graphene through electrochemical exfoliation and its utilization as electrodes for zinc bromine redox flow battery. J. Electrochem. Soc. 2016, 163, A2899–A2910. [Google Scholar] [CrossRef]
- Yuan, Z.; Peng, H.J.; Hou, T.Z.; Huang, J.Q.; Chen, C.M.; Wang, D.W.; Cheng, X.B.; Wei, F.; Zhang, Q. Powering lithium–sulfur battery performance by propelling polysulfide redox at sulfiphilic hosts. Nano Lett. 2016, 16, 519–527. [Google Scholar] [CrossRef]
- Babu, G.; Ababtain, K.; Ng, K.S.; Arava, L.M.R. Electrocatalysis of lithium polysulfides: Current collectors as electrodes in Li/S battery configuration. Sci. Rep. 2015, 5, 8763. [Google Scholar] [CrossRef] [Green Version]
- Al Salem, H.; Babu, G.; Rao, C.V.; Arava, L.M. Electrocatalytic Polysulfide Traps for Controlling Redox Shuttle Process of Li-S Batteries. J. Am. Chem. Soc. 2015, 137, 11542–11545. [Google Scholar] [CrossRef] [PubMed]
- Babu, G.; Masurkar, N.; Al Salem, H.; Arava, L.M. Transition Metal Dichalcogenide Atomic Layers for Lithium Polysulfides Electrocatalysis. J. Am. Chem. Soc. 2017, 139, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Ong, S.P.; Hautier, G.; Chen, W.; Richards, W.D.; Dacek, S.; Cholia, S.; Gunter, D.; Skinner, D.; Ceder, G. Commentary: The Materials Project: A materials genome approach to accelerating materials innovation. APL Mater. 2013, 1, 011002. [Google Scholar] [CrossRef] [Green Version]
- Ong, S.P.; Richards, W.D.; Jain, A.; Hautier, G.; Kocher, M.; Cholia, S.; Gunter, D.; Chevrier, V.L.; Persson, K.A.; Ceder, G. Python Materials Genomics (pymatgen): A robust, open-source python library for materials analysis. Comput. Mater. Sci. 2013, 68, 314–319. [Google Scholar] [CrossRef] [Green Version]
- Momma, K.; Izumi, F. VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Cryst. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
- Reddy, A.; Shaijumon, M.; Rajalakshmi, N.; Ramaprabhu, S. Performance of proton exchange membrane fuel cells using Pt/MWNT–Pt/C composites as electrocatalysts for oxygen reduction reaction in proton exchange membrane fuel cells. J. Fuel Cell Sci. Technol. 2010, 7, 021001. [Google Scholar] [CrossRef]
- Mosavati, N.; Chitturi, V.R.; Arava, L.M.R.; Salley, S.O.; Ng, K.S. Effects of Nickel Particle Size and Graphene Support on the Electrochemical Performance of Lithium/Dissolved Polysulfide Batteries. Electrochim. Acta 2015, 185, 297–303. [Google Scholar] [CrossRef] [Green Version]
- Thangavel, N.K.; Gopalakrishnan, D.; Arava, L.M.R. Understanding Heterogeneous Electrocatalysis of Lithium Polysulfide Redox on Pt and WS2 Surfaces. J. Phys. Chem. C 2017, 121, 12718–12725. [Google Scholar] [CrossRef]
- Fan, C.Y.; Xiao, P.; Li, H.H.; Wang, H.F.; Zhang, L.L.; Sun, H.Z.; Wu, X.L.; Xie, H.M.; Zhang, J.P. Nanoscale Polysulfides Reactors Achieved by Chemical Au-S Interaction: Improving the Performance of Li-S Batteries on the Electrode Level. ACS Appl. Mater. Interfaces 2015, 7, 27959–27967. [Google Scholar] [CrossRef]
- Sawas, A.; Babu, G.; Thangavel, N.K.; Arava, L.M.R. Electrocatalysis driven high energy density Li-ion polysulfide battery. Electrochim. Acta 2019, 307, 253–259. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, W.; Zhou, D.; Tian, H.; Su, D.; Wang, C.; Stockdale, D.; Kang, F.; Li, B.; Wang, G. Co–Fe Mixed Metal Phosphide Nanocubes with Highly Interconnected-Pore Architecture as an Efficient Polysulfide Mediator for Lithium–Sulfur Batteries. ACS Nano 2019, 13, 4731–4741. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, C.; Wang, W.; Eng, A.Y.S.; Wan, M.; Fu, L.; Mao, E.; Li, G.; Tang, J.; Seh, Z.W. Enhanced Chemical Immobilization and Catalytic Conversion of Polysulfide Intermediates Using Metallic Mo Nanoclusters for High-Performance Li-S Batteries. ACS Nano 2019, 14, 1148–1157. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Niu, S.; Lv, W.; Zhou, G.; Li, J.; Fan, S.; Deng, Y.; Pan, Z.; Li, B.; Kang, F. Propelling polysulfides transformation for high-rate and long-life lithium–sulfur batteries. Nano Energy 2017, 33, 306–312. [Google Scholar] [CrossRef] [Green Version]
- Ma, L.; Chen, R.; Zhu, G.; Hu, Y.; Wang, Y.; Chen, T.; Liu, J.; Jin, Z. Cerium oxide nanocrystal embedded bimodal micromesoporous nitrogen-rich carbon nanospheres as effective sulfur host for lithium–sulfur batteries. ACS Nano 2017, 11, 7274–7283. [Google Scholar] [CrossRef]
- Guo, B.; Bandaru, S.; Dai, C.; Chen, H.; Zhang, Y.; Xu, Q.; Bao, S.; Chen, M.; Xu, M. Self-Supported FeCo2S4 Nanotube Arrays as Binder-Free Cathodes for Lithium–Sulfur Batteries. ACS Appl. Mater. Interfaces 2018, 10, 43707–43715. [Google Scholar] [CrossRef]
- Al Salem, H.; Chitturi, V.R.; Babu, G.; Santana, J.A.; Gopalakrishnan, D.; Arava, L.M.R. Stabilizing polysulfide-shuttle in a Li–S battery using transition metal carbide nanostructures. RSC Adv. 2016, 6, 110301–110306. [Google Scholar] [CrossRef]
- Zhou, F.; Li, Z.; Luo, X.; Wu, T.; Jiang, B.; Lu, L.L.; Yao, H.B.; Antonietti, M.; Yu, S.H. Low cost metal carbide nanocrystals as binding and electrocatalytic sites for high performance Li–S batteries. Nano Lett. 2018, 18, 1035–1043. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.; Garapati, M.S.; Vijaya Kumar Saroja, A.P.; Sundara, R. Polar Bilayer Cathode for Advanced Lithium–Sulfur Battery: Synergy Between Polysulfide Conversion and Confinement. J. Phys. Chem. C 2019, 123, 10777–10787. [Google Scholar] [CrossRef]
- Liang, X.; Hart, C.; Pang, Q.; Garsuch, A.; Weiss, T.; Nazar, L.F. A highly efficient polysulfide mediator for lithium–sulfur batteries. Nat. Commun. 2015, 6, 5682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imtiaz, S.; Ali Zafar, Z.; Razaq, R.; Sun, D.; Xin, Y.; Li, Q.; Zhang, Z.; Zheng, L.; Huang, Y.; Anderson, J.A. Electrocatalysis on Separator Modified by Molybdenum Trioxide Nanobelts for Lithium–Sulfur Batteries. Adv. Mater. Interfaces 2018, 5, 1800243. [Google Scholar] [CrossRef]
- Lin, H.; Zhang, S.; Zhang, T.; Ye, H.; Yao, Q.; Zheng, G.W.; Lee, J.Y. Elucidating the Catalytic Activity of Oxygen Deficiency in the Polysulfide Conversion Reactions of Lithium–Sulfur Batteries. Adv. Energy Mater. 2018, 8, 1801868. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, Y.; Zhang, Y.; Xiang, M.; Wu, H.; Liu, H.; Dou, S. Interwoven V2O5 nanowire/graphene nanoscroll hybrid assembled as efficient polysulfide-trapping-conversion interlayer for long-life lithium–sulfur batteries. J. Mater. Chem. A 2018, 6, 19358–19370. [Google Scholar] [CrossRef]
- Hu, N.; Lv, X.; Dai, Y.; Fan, L.; Xiong, D.; Li, X. SnO2/Reduced Graphene Oxide Interlayer Mitigating the Shuttle Effect of Li–S Batteries. ACS Appl. Mater. Interfaces 2018, 10, 18665–18674. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.Y.; Kim, H.M.; Lee, S.K.; Lee, J.H.; Abouimrane, A.; Khaleel, M.A.; Belharouak, I.; Manthiram, A.; Sun, Y.K. High-Energy, High-Rate, Lithium–Sulfur Batteries: Synergetic Effect of Hollow TiO2-Webbed Carbon Nanotubes and a Dual Functional Carbon-Paper Interlayer. Adv. Energy Mater. 2016, 6, 1501480. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhou, C.; Li, Q.; Yan, C.; Han, B.; Xia, K.; Gao, Q.; Wu, J. Enabling Prominent High-Rate and Cycle Performances in One Lithium–Sulfur Battery: Designing Permselective Gateways for Li+ Transportation in Holey-CNT/S Cathodes. Adv. Mater. 2015, 27, 3774–3781. [Google Scholar] [CrossRef] [PubMed]
- Hao, Z.; Zeng, R.; Yuan, L.; Bing, Q.; Liu, J.; Xiang, J.; Huang, Y. Perovskite La0.6Sr0.4CoO3-δ as a new polysulfide immobilizer for high-energy lithium-sulfur batteries. Nano Energy 2017, 40, 360–368. [Google Scholar] [CrossRef]
- Bao, J.; Zhang, X.; Fan, B.; Zhang, J.; Zhou, M.; Yang, W.; Hu, X.; Wang, H.; Pan, B.; Xie, Y. Ultrathin Spinel-Structured Nanosheets Rich in Oxygen Deficiencies for Enhanced Electrocatalytic Water Oxidation. Angew. Chem. Int. Ed. 2015, 54, 7399–7404. [Google Scholar] [CrossRef]
- Gao, R.; Liu, L.; Hu, Z.; Zhang, P.; Cao, X.; Wang, B.; Liu, X. The role of oxygen vacancies in improving the performance of CoO as a bifunctional cathode catalyst for rechargeable Li–O2 batteries. J. Mater. Chem. A 2015, 3, 17598–17605. [Google Scholar] [CrossRef]
- Bruix, A.; Füchtbauer, H.G.; Tuxen, A.K.; Walton, A.S.; Andersen, M.; Porsgaard, S.; Besenbacher, F.; Hammer, B.; Lauritsen, J.V. In Situ Detection of Active Edge Sites in Single-Layer MoS2 Catalysts. ACS Nano 2015, 9, 9322–9330. [Google Scholar] [CrossRef] [Green Version]
- Bonaccorso, F.; Colombo, L.; Yu, G.; Stoller, M.; Tozzini, V.; Ferrari, A.C.; Ruoff, R.S.; Pellegrini, V. Graphene, related two-dimensional crystals, and hybrid systems for energy conversion and storage. Science 2015, 347, 1246501. [Google Scholar] [CrossRef]
- Masurkar, N.; Thangavel, N.K.; Arava, L.M.R. CVD-Grown MoSe2 Nanoflowers with Dual Active Sites for Efficient Electrochemical Hydrogen Evolution Reaction. ACS Appl. Mater. Interfaces 2018, 10, 27771–27779. [Google Scholar] [CrossRef]
- Chhowalla, M.; Shin, H.S.; Eda, G.; Li, L.J.; Loh, K.P.; Zhang, H. The chemistry of two-dimensional layered transition metal dichalcogenide nanosheets. Nat. Chem. 2013, 5, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Zhang, C.; Zhou, G.; Lv, W.; Ling, G.; Zhi, L.; Yang, Q.H. Catalytic Effects in Lithium–Sulfur Batteries: Promoted Sulfur Transformation and Reduced Shuttle Effect. Adv. Sci. 2018, 5, 1700270. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Yang, S.; Li, B. Ultrathin bismuth nanosheets as an efficient polysulfide catalyst for high performance lithium–sulfur batteries. J. Mater. Chem. A 2020, 8, 149–157. [Google Scholar] [CrossRef]
- Wang, L.; Song, Y.H.; Zhang, B.H.; Liu, Y.T.; Wang, Z.Y.; Li, G.R.; Liu, S.; Gao, X.P. Spherical Metal Oxides with High Tap Density as Sulfur Host to Enhance Cathode Volumetric Capacity for Lithium-Sulfur Battery. ACS Appl. Mater. Interfaces 2020, 12, 5909–5919. [Google Scholar] [CrossRef]
- Boyjoo, Y.; Shi, H.; Olsson, E.; Cai, Q.; Wu, Z.S.; Liu, J.; Lu, G.Q. Molecular-Level Design of Pyrrhotite Electrocatalyst Decorated Hierarchical Porous Carbon Spheres as Nanoreactors for Lithium–Sulfur Batteries. Adv. Energy Mater. 2020, 10, 2000651. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Zhang, S.; Zhang, J.; Xu, M.; Tatara, R.; Dokko, K.; Watanabe, M. Three-Dimensionally Hierarchical Ni/Ni3S2/S Cathode for Lithium-Sulfur Battery. ACS Appl. Mater. Interfaces 2017, 9, 38477–38485. [Google Scholar] [CrossRef] [Green Version]
- Pu, J.; Shen, Z.; Zheng, J.; Wu, W.; Zhu, C.; Zhou, Q.; Zhang, H.; Pan, F. Multifunctional Co3S4@ sulfur nanotubes for enhanced lithium-sulfur battery performance. Nano Energy 2017, 37, 7–14. [Google Scholar] [CrossRef]
- He, J.; Hartmann, G.; Lee, M.; Hwang, G.S.; Chen, Y.; Manthiram, A. Freestanding 1T MoS2/graphene heterostructures as a highly efficient electrocatalyst for lithium polysulfides in Li–S batteries. Energy Environ. Sci. 2019, 12, 344–350. [Google Scholar] [CrossRef]
- Hu, L.; Dai, C.; Lim, J.M.; Chen, Y.; Lian, X.; Wang, M.; Li, Y.; Xiao, P.; Henkelman, G.; Xu, M. A highly efficient double-hierarchical sulfur host for advanced lithium-sulfur batteries. Chem. Sci. 2018, 9, 666–675. [Google Scholar] [CrossRef] [Green Version]
- Luo, L.; Chung, S.H.; Yaghoobnejad Asl, H.; Manthiram, A. Long-Life Lithium-Sulfur Batteries with a Bifunctional Cathode Substrate Configured with Boron Carbide Nanowires. Adv. Mater. 2018, 30, e1804149. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Chen, Y.; Manthiram, A. Vertical Co9S8 hollow nanowall arrays grown on a Celgard separator as a multifunctional polysulfide barrier for high-performance Li–S batteries. Energy Environ. Sci. 2018, 11, 2560–2568. [Google Scholar] [CrossRef]
- Chen, T.; Ma, L.; Cheng, B.; Chen, R.; Hu, Y.; Zhu, G.; Wang, Y.; Liang, J.; Tie, Z.; Liu, J. Metallic and polar Co9S8 inlaid carbon hollow nanopolyhedra as efficient polysulfide mediator for lithium−sulfur batteries. Nano Energy 2017, 38, 239–248. [Google Scholar] [CrossRef]
- Lin, H.; Yang, L.; Jiang, X.; Li, G.; Zhang, T.; Yao, Q.; Zheng, G.W.; Lee, J.Y. Electrocatalysis of polysulfide conversion by sulfur-deficient MoS2 nanoflakes for lithium–sulfur batteries. Energy Environ. Sci. 2017, 10, 1476–1486. [Google Scholar] [CrossRef] [Green Version]
- Gao, X.; Yang, X.; Li, M.; Sun, Q.; Liang, J.; Luo, J.; Wang, J.; Li, W.; Liang, J.; Liu, Y.; et al. Cobalt-Doped SnS2 with Dual Active Centers of Synergistic Absorption-Catalysis Effect for High-S Loading Li-S Batteries. Adv. Funct. Mater. 2019, 29, 1806724. [Google Scholar] [CrossRef]
- Zhou, G.; Tian, H.; Jin, Y.; Tao, X.; Liu, B.; Zhang, R.; Seh, Z.W.; Zhuo, D.; Liu, Y.; Sun, J.; et al. Catalytic oxidation of Li2S on the surface of metal sulfides for Li–S batteries. Proc. Nat. Acad. Sci. USA 2017, 114, 840–845. [Google Scholar] [CrossRef] [Green Version]
- Lu, X.; Zhang, Q.; Wang, J.; Chen, S.; Ge, J.; Liu, Z.; Wang, L.; Ding, H.; Gong, D.; Yang, H.; et al. High performance bimetal sulfides for lithium-sulfur batteries. Chem. Eng. J. 2019, 358, 955–961. [Google Scholar] [CrossRef]
- Zhong, Y.; Xia, X.; Shi, F.; Zhan, J.; Tu, J.; Fan, H.J. Transition Metal Carbides and Nitrides in Energy Storage and Conversion. Adv. Sci. 2016, 3, 1500286. [Google Scholar] [CrossRef]
- Ham, D.J.; Lee, J.S. Transition metal carbides and nitrides as electrode materials for low temperature fuel cells. Energies 2009, 2, 873–899. [Google Scholar] [CrossRef]
- Peng, H.J.; Zhang, G.; Chen, X.; Zhang, Z.W.; Xu, W.T.; Huang, J.Q.; Zhang, Q. Enhanced electrochemical kinetics on conductive polar mediators for lithium–sulfur batteries. Angew. Chem. Int. Ed. 2016, 55, 12990–12995. [Google Scholar] [CrossRef]
- Bao, W.; Su, D.; Zhang, W.; Guo, X.; Wang, G. 3D Metal Carbide@ Mesoporous Carbon Hybrid Architecture as a New Polysulfide Reservoir for Lithium-Sulfur Batteries. Adv. Funct. Mater. 2016, 26, 8746–8756. [Google Scholar] [CrossRef]
- Pourali, Z.; Yaftian, M.R.; Sovizi, M.R. Li2S/transition metal carbide composite as cathode material for high performance lithium-sulfur batteries. Mater. Chem. Phys. 2018, 217, 117–124. [Google Scholar] [CrossRef]
- Liang, X.; Garsuch, A.; Nazar, L.F. Sulfur cathodes based on conductive MXene nanosheets for high-performance lithium-sulfur batteries. Angew. Chem. Int. Ed. 2015, 54, 3907–3911. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.; Chen, X.; Hu, W.; Chuang, C.; Xie, S.; Hu, A.; Yan, W.; Kong, X.; Wu, X.; Ji, H. Cobalt in nitrogen-doped graphene as single-atom catalyst for high-sulfur content lithium–sulfur batteries. J. Am. Chem. Soc. 2019, 141, 3977–3985. [Google Scholar] [CrossRef] [PubMed]
- Lim, W.G.; Mun, Y.; Cho, A.; Jo, C.; Lee, S.; Han, J.W.; Lee, J. Synergistic Effect of Molecular-Type Electrocatalysts with Ultrahigh Pore Volume Carbon Microspheres for Lithium-Sulfur Batteries. ACS Nano 2018, 12, 6013–6022. [Google Scholar] [CrossRef]
- Wang, N.; Chen, B.; Qin, K.; Liu, E.; Shi, C.; He, C.; Zhao, N. Rational design of Co9S8/CoO heterostructures with well-defined interfaces for lithium sulfur batteries: A study of synergistic adsorption-electrocatalysis function. Nano Energy 2019, 60, 332–339. [Google Scholar] [CrossRef]
- Wei, Y.; Kong, Z.; Pan, Y.; Cao, Y.; Long, D.; Wang, J.; Qiao, W.; Ling, L. Sulfur film sandwiched between few-layered MoS2 electrocatalysts and conductive reduced graphene oxide as a robust cathode for advanced lithium–sulfur batteries. J. Mater. Chem. A 2018, 6, 5899–5909. [Google Scholar] [CrossRef]
- Zhou, H.Y.; Sui, Z.Y.; Amin, K.; Lin, L.W.; Wang, H.Y.; Han, B.H. Investigating the Electrocatalysis of a Ti3C2/Carbon Hybrid in Polysulfide Conversion of Lithium-Sulfur Batteries. ACS Appl. Mater. Interfaces 2020, 12, 13904–13913. [Google Scholar] [CrossRef]
- Wu, Y.; Deng, J.; Zhou, Y.; Huang, Y.; Li, Y. Molybdenum carbide nanostructures for electrocatalytic polysulfide conversion in lithium-polysulfide batteries. Nanoscale Horiz. 2020, 5, 501–506. [Google Scholar] [CrossRef]
- Razaq, R.; Sun, D.; Xin, Y.; Li, Q.; Huang, T.; Zheng, L.; Zhang, Z.; Huang, Y. Enhanced kinetics of polysulfide redox reactions on Mo2C/CNT in lithium-sulfur batteries. Nanotechnology 2018, 29, 295401. [Google Scholar] [CrossRef]
- Cheng, X.B.; Huang, J.Q.; Zhang, Q. Li metal anode in working lithium-sulfur batteries. J. Electrochem. Soc. 2017, 165, A6058. [Google Scholar] [CrossRef]
- Gruber, P.W.; Medina, P.A.; Keoleian, G.A.; Kesler, S.E.; Everson, M.P.; Wallington, T.J. Global lithium availability: A constraint for electric vehicles? J. Ind. Ecol. 2011, 15, 760–775. [Google Scholar] [CrossRef]
- Swain, B. Recovery and recycling of lithium: A review. Sep. Purif. Technol. 2017, 172, 388–403. [Google Scholar] [CrossRef]
- Kavanagh, L.; Keohane, J.; Garcia Cabellos, G.; Lloyd, A.; Cleary, J. Global lithium sources—Industrial use and future in the electric vehicle industry: A review. Resources 2018, 7, 57. [Google Scholar] [CrossRef] [Green Version]
- Tran, M.K.; Rodrigues, M.T.F.; Kato, K.; Babu, G.; Ajayan, P.M. Deep eutectic solvents for cathode recycling of Li-ion batteries. Nat. Energy 2019, 4, 339–345. [Google Scholar] [CrossRef]
- Miroshnikov, M.; Kato, K.; Babu, G.; Thangavel, N.K.; Mahankali, K.; Hohenstein, E.; Wang, H.; Satapathy, S.; Divya, K.P.; Asare, H.; et al. Made From Henna! A Fast-Charging, High-Capacity, and Recyclable Tetrakislawsone Cathode Material for Lithium Ion Batteries. ACS Sustain. Chem. Eng. 2019, 7, 13836–13844. [Google Scholar] [CrossRef]










| Control Material and Electrocatalytic Electrode | Cathodic Exchange Current Density (mA cm−2) | Anodic Exchange Current Density (mA cm−2) | Cathodic Peak Position for Li2S8→Li2S4 (V vs. Li/Li+) | Cathodic Peak Position for Li2S4→Li2S/Li2S2 (V vs. Li/Li+) | Ref. |
|---|---|---|---|---|---|
| Carbon Ni | 0.049 0.071 | - - | 2.40 2.43 | 1.84 1.94 | [60] |
| Graphene Pt on graphene | 1.18 3.18 | 0.17 0.25 | 2.42 2.45 | 1.93 1.96 | [61] |
| CNF CNT + CNF Mo + CNT+ CNF | 24 × 10−3 35 × 10−3 75 × 10−3 | - | - | ~2.07 ~2.08 2.11 | [72] |
| PG Fe2O3 on PG | 2.28 3.46 | 4.81 4.96 | 2.32 2.35 | 2.03 2.04 | [73] |
| NC CeO2 on NC | - - | - - | ~2.22 2.27 | ~1.95 2.01 | [74] |
| Carbon WS2 | 8.5 × 10−3 11.8 × 10−3 | - - | 2.21 2.24 | 1.67 1.78 | [62] |
| Graphene CoS2/graphene | - | - | 2.09 2.25 | 1.81 2.00 | [59] |
| Carbon cloth FeCo2S4 | - | - | 2.30 2.32 | 2.05 1.98 | [75] |
| Carbon TiC | - | - | 2.38 2.45 | 1.91 1.95 | [76] |
| CNF W2C-CNF | - | - | 2.35 2.41 | 2.04 2.08 | [77] |
| NS-PC TiC-NS-PC | 31.28 × 10−3 42.35 × 10−3 | 9.29 × 10−3 12.65 × 10−3 | 2.27 2.34 | - | [78] |
| Material | Li2S (eV) | Li2S4 (eV) | Li2S6 (eV) | Li2S8 (eV) | S8 (eV) | Ref. | |
|---|---|---|---|---|---|---|---|
| Non-metal | Graphene | 0.65 | 0.72 | 0.93 | 1.10 | 0.89 | [73] |
| Metal | Gold nanoparticles | 1.81 | - | - | - | - | [69] |
| Co-Fe-P | - | - | −3.92 (Co-S) −7.40 (FeP2) | - | - | [71] | |
| Bismuth | −2.36 | −0.45 | −0.32 | −0.39 | - | [94] | |
| Metal oxides | Fe2O3 | 4.85 | 4.09 | 4.11 | 3.78 | 2.04 | [73] |
| Ceo2 | −1.96 | −2.90 | −5.48 | −5.63 | −2.42 | [74] | |
| LiNi0.8Co0.1Mn0.1O2 | - | - | −2.25 | - | - | [95] | |
| Metal Sulfides | CoS2 | - | 1.97 | - | - | - | [59] |
| Fe7S8 | - | −4.25 | −4.33 | −5.00 | - | [96] | |
| Ni3S2 | 4.89 | 2.29 | 2.15 | 1.92 | 1.09 | [97] | |
| Co3S4 | - | 2.26 | 1.61 | 1.68 | - | [98] | |
| 1T-MoS2 | ~1.25 | ~1.15 | ~1.30 | ~1.45 | ~1.28 | [99] | |
| MoS2 | 0.87 | 0.32 | 0.22 | 0.10 | 0.05 | [100] | |
| FeCo2S4 | −6.61 | −4.50 | −3.94 | −5.21 | - | [75] | |
| Metal Carbides | TiC | - | - | - | 3.68 | - | [76] |
| TiC-N-S-C | −3.80 | −4.00 | −2.00 | −3.50 | - | [78] | |
| W2C | - | - | −2.57 | - | - | [77] | |
| B4C (100) facet | - | 12.51 | - | - | -- | [101] |
| Electrocatalyst | Sulfur Loading (mg cm−2) | C-Rate | Discharge Capacity (mAh g−1) | Cycles | CE (%) | Ref. | |
|---|---|---|---|---|---|---|---|
| Metals | Co-Fe-P | 1 | 0.2 | 1118 | 100 | 100 | [71] |
| 1 | 1 | 863 | 500 | 100 | |||
| 3.7 | 0.2 | ~1100 | 100 | ~100 | |||
| 5.5 | 0.2 | ~890 | 100 | ~100 | |||
| Mo nanoclusters | 1.91 | 1 | ~1100 | 500 | 99.6 | [72] | |
| 7.64 | 0.2 | ~800 | 100 | - | |||
| Co in Nitrogen doped graphene | 2 | 1 | 866 | 500 | ~99.6 | [114] | |
| 6 | 0.2 | ~5.1 mAh cm−2 | 100 | - | |||
| Fe-N-C | 2.5 | 0.5 | 1631 | 100 | 95 | [115] | |
| 5.2 | 3 | 483 | 500 | - | |||
| Metal oxides | LiNi0.8Co0.1Mn0.1O2 | 0.7 | 0.1 | 1264.3 | 500 | 99.22 | [95] |
| 4.29 | 0.1 | ~700 | 120 | - | |||
| Co9S8-CoO | 1 | 1 | 956 | 300 | ~100 | [116] | |
| 2.5 | 1 | 925 | 1000 | ~100 | |||
| Metal sulfides | Fe1−xS | - | 0.5 | 1070 | 200 | ~100 | [96] |
| - | 1 | 793 | 200 | - | |||
| 8.14 | 0.05 | 7.4 mAh cm−2 | 60 | - | |||
| Ni3S2 | 4 | 1 mA cm−2 | 655 | 80 | ~95 | [97] | |
| 4 | 4 mA cm−2 | 441 | 150 | - | |||
| MoS2 | 1 | 0.2 | 954 | 150 | 99.5 | [117] | |
| 1 | 2 | ~750 | 1000 | ~100 | |||
| 3.6 | 0.2 | 714 | 110 | - | |||
| Metal carbides | Ti3C2 | 1.2–1.5 | 0.5 | 1180 | 200 | 99 | [118] |
| 1.2–1.5 | 1 | 530 | 500 | ~100 | |||
| 1 | 1 | 610 | 200 | ~100 | |||
| 2.5 | 1 | 475 | 200 | ~100 | |||
| MoC1−x | 2 | 800 mA g−1 | 1000 | 500 | ~98 | [119] | |
| 2 | 1600 mA g−1 | 900 | 200 | - | |||
| 4 | 1.6 mA cm−2 | 2.6 mAh cm−2 | 100 | 88 | |||
| 6 | 1.6 mA cm−2 | 3.6 mAh cm−2 | 100 | 91 | |||
| Mo2C | 1.5–1.8 | 0.5 | 1206 | 100 | ~100 | [120] | |
| 1.5–1.8 | 2 | 802 | 900 | ~100 | |||
| 2.5 | 1 | 835 | 100 | ~100 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahankali, K.; Nagarajan, S.; Thangavel, N.K.; Rajendran, S.; Yeddala, M.; Arava, L.M.R. Metal-Based Electrocatalysts for High-Performance Lithium-Sulfur Batteries: A Review. Catalysts 2020, 10, 1137. https://doi.org/10.3390/catal10101137
Mahankali K, Nagarajan S, Thangavel NK, Rajendran S, Yeddala M, Arava LMR. Metal-Based Electrocatalysts for High-Performance Lithium-Sulfur Batteries: A Review. Catalysts. 2020; 10(10):1137. https://doi.org/10.3390/catal10101137
Chicago/Turabian StyleMahankali, Kiran, Sudhan Nagarajan, Naresh Kumar Thangavel, Sathish Rajendran, Munaiah Yeddala, and Leela Mohana Reddy Arava. 2020. "Metal-Based Electrocatalysts for High-Performance Lithium-Sulfur Batteries: A Review" Catalysts 10, no. 10: 1137. https://doi.org/10.3390/catal10101137