Partial Versus Total Omentectomy in Patients with Gastric Cancer: A Systemic Review and Meta-Analysis
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Literature Search Strategy
2.2. Data Extraction
2.3. Inclusion and Exclusion Criteria
2.4. Assessment of The Quality of The Methods
2.5. Statistical Analyses
3. Results
3.1. Included Trials
3.2. Effects of the intervention
3.2.1. Primary outcomes
3.2.2. Secondary Outcomes
3.2.3. Additional Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- International Agency for Research on Cancer. Gatric Cancer Factsheet 2020. Available online: https://gco.iarc.fr/today/home (accessed on 12 June 2021).
- Xiong, B.; Ma, L.; Huang, W.; Cheng, Y.; Luo, H.; Wang, K. Efficiency of bursectomy in patients with resectable gastric cancer: An updated meta–Analysis. Eur. J. Surg. Oncol. 2019, 45, 1483–1492. [Google Scholar] [CrossRef]
- Hagiwara, A.; Takahashi, T.; Sawai, K.; Taniguchi, H.; Shimotsuma, M.; Okano, S.; Sakakura, C.; Tsujimoto, H.; Osaki, K.; Sasaki, S.; et al. Milky spots as the implantation site for malignant cells in peritoneal dissemination in mice. Cancer Res. 1993, 53, 687–692. [Google Scholar]
- Kodera, Y.; Nakanishi, H.; Ito, S.; Yamamura, Y.; Kanemitsu, Y.; Shimizu, Y.; Hirai, T.; Yasui, K.; Kato, T.; Tatematsu, M. Quantitative detection of disseminated cancer cells in the greater omentum of gastric carcinoma patients with real–time RT–PCR: A comparison with peritoneal lavage cytology. Gastric Cancer 2002, 5, 69–76. [Google Scholar] [CrossRef] [Green Version]
- Japenese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2018. Gastric Cancer 2021, 24, 1–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smyth, E.; Verheij, M.; Allum, W.; Cunningham, D.; Cervantes, A.; Arnold, D. Gastric cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow–up. Ann. Oncol. 2016, 27, v38–v49. [Google Scholar] [CrossRef]
- Karabulut, B.; Sönmez, K.; Türkyılmaz, Z.; Demiroğulları, B.; Karabulut, R.; Sezer, C.; Sultan, N.; Başaklar, A.C.; Kale, N. Omentum prevents intestinal adhesions to mesh graft in abdominal infections and serosal defects. Surg. Endosc. 2006, 20, 978–982. [Google Scholar] [CrossRef] [PubMed]
- Liebermann–Meffert, D. The greater omentum: Anatomy, embryology, and surgical applications. Surg. Clin. N. Am. 2000, 80, 275–293. [Google Scholar] [CrossRef]
- Platell, C.; Cooper, D.; Papadimitriou, J.M.; Hall, J.C. The omentum. World J. Gastroenterol. 2000, 6, 169. [Google Scholar]
- Higgins, J.P.; Altman, D.G.; Gotzsche, P.C.; Juni, P.; Moher, D.; Oxman, A.D.; Savovic, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.; et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef] [Green Version]
- Wells, G.; Shea, B.; O’Connell, D.; Peterson, j.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle–Ottawa Scale (NOS) for Assessing the Quality of Non–Randomized Studies in Meta–Analysis. 2013. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 7 May 2021).
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gotzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta–analyses of studies that evaluate health care interventions: Explanation and elaboration. J. Clin. Epidemiol. 2009, 62, e1–e34. [Google Scholar] [CrossRef] [Green Version]
- Tierney, J.F.; Stewart, L.A.; Ghersi, D.; Burdett, S.; Sydes, M.R. Practical methods for incorporating summary time–to–event data into meta–analysis. Trials 2007, 8, 16. [Google Scholar] [CrossRef] [Green Version]
- Hozo, S.P.; Djulbegovic, B.; Hozo, I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med. Res. Methodol. 2005, 5, 13. [Google Scholar] [CrossRef] [Green Version]
- Balduzzi, S.; Rücker, G.; Schwarzer, G. How to perform a meta–analysis with R: A practical tutorial. Evid.–Based Ment. Health 2019, 22, 153–160. [Google Scholar] [CrossRef] [Green Version]
- Viechtbauer, W. Conducting Meta–Analyses in R with The metafor Package. J. Stat. Software. 2010, 36, 1–48. [Google Scholar] [CrossRef] [Green Version]
- Sarkar, D. Lattice: Multivariate Data Visualization with R; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2008; pp. 98–101. [Google Scholar]
- Ha, T.K.; An, J.Y.; Youn, H.G.; Noh, J.H.; Sohn, T.S.; Kim, S. Omentum—Preserving gastrectomy for early gastric cancer. World J. Surg. 2008, 32, 1703–1708. [Google Scholar] [CrossRef]
- Kim, J.H.; Kang, S.H.; Oh, S.T.; Yook, J.H.; Kim, B.S.; Park, K.C. Following of the Omentum Preserving Gastrectomy for Advanced Gastric Cancer without Serosa Exposure. J. Korean Surg. Soc. 2009, 76, 154–158. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.C.; Kim, K.H.; Jung, G.J.; Rattner, D.W. Comparative study of complete and partial omentectomy in radical subtotal gastrectomy for early gastric cancer. Yonsei Med. J. 2011, 52, 961–966. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasegawa, S.; Yamamoto, Y.; Taguri, M.; Morita, S.; Sato, T.; Yamada, R.; Oshima, T.; Yukawa, N.; Yoshikawa, T.; Rino, Y.; et al. A randomized phase II trial of omentum–preserving gastrectomy for advanced gastric cancer. Jpn. J. Clin. Oncol. 2013, 43, 214–216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, D.J.; Lee, J.H.; Kim, W. A comparison of total versus partial omentectomy for advanced gastric cancer in laparoscopic gastrectomy. World J. Surg. Oncol. 2014, 12, 64. [Google Scholar] [CrossRef] [Green Version]
- Ri, M.; Nunobe, S.; Honda, M.; Akimoto, E.; Kinoshita, T.; Hori, S.; Aizawa, M.; Yabusaki, H.; Isobe, Y.; Kawakubo, H.; et al. Gastrectomy with or without omentectomy for cT3–4 gastric cancer: A multicentre cohort study. Br. J. Surg. 2020, 107, 1640–1647. [Google Scholar] [CrossRef]
- Sakimura, Y.; Inaki, N.; Tsuji, T.; Kadoya, S.; Bando, H. Long–term outcomes of omentum–preserving versus resecting gastrectomy for locally advanced gastric cancer with propensity score analysis. Sci. Rep. 2020, 10, 16305. [Google Scholar] [CrossRef]
- Murakami, H.; Yamada, T.; Taguri, M.; Hasegawa, S.; Yamanaka, T.; Rino, Y.; Mushiake, H.; Oshima, T.; Matsukawa, H.; Tani, K.; et al. Short–Term Outcomes from a Randomized Screening Phase II Non–inferiority Trial Comparing Omentectomy and Omentum Preservation for Locally Advanced Gastric Cancer: The TOP–G Trial. World J. Surg. 2021, 45, 1803–1811. [Google Scholar] [CrossRef]
- Seo, W.J.; Choi, S.; Roh, C.K.; Cho, M.; Kim, Y.M.; Kim, H.I.; Hyung, W.J. Omentum preservation as an oncologically comparable and surgically superior alternative to total omentectomy during radical gastrectomy for T3–T4 gastric cancer. Surgery 2012, in press. [Google Scholar]
- Ishizuka, M.; Shibuya, N.; Takagi, K.; Hachiya, H.; Tago, K.; Matsumoto, T.; Shimizu, T.; Aoki, T.; Kubota, K. Omentectomy Does Not Affect the Postoperative Outcome of Patients with Locally Advanced Gastric Cancer: A Systematic Review and Meta–Analysis. J. Surg. Res. 2021, 264, 287–295. [Google Scholar] [CrossRef]
- Fujita, J.; Tsukahara, Y.; Ikeda, K.; Akagi, K.; Kan, K.; Hata, S.; Fukushima, Y.; Shibata, T.; Kitada, M.; Shimano, T. Evaluation of omentum preserving gastrectomy for advanced gastric cancer. Jpn. J. Gastroenterol. Surg. 2003, 36, 1151–1158. [Google Scholar] [CrossRef]
- Watanabe, N.; Nashimoto, A.; Yabusaki, H.; Takii, Y.; Tsuchiya, Y.; Tanaka, O. Evaluation of omento–bursectomy for T2 and T3 gastric cancer. J. Jpn. Surg. Assoc. 2004, 65, 2570–2574. [Google Scholar] [CrossRef]
- Yamamura, Y.; Ito, S.; Mochizuki, Y.; Kanemitsu, Y.; Shimizu, Y.; Hirai, T. Studies on omentectomy and bursectomy for surgical treatment of gastric cancer. Gekachiryo 2004, 90, 70–76. [Google Scholar]
- Fujitani, K.; Kurokawa, Y.; Takeno, A.; Endoh, S.; Ohmori, T.; Fujita, J.; Yamasaki, M.; Takiguchi, S.; Mori, M.; Doki, Y.; et al. Time to initiation or duration of S-1 adjuvant chemotherapy; which really impacts on survival in stage II and III gastric cancer? Gastric Cancer 2018, 21, 446–452. [Google Scholar] [CrossRef] [Green Version]
- Radulescu, D.; Baleanu, V.D.; Padureanu, V.; Radulescu, P.M.; Bordu, S.; Patrascu, S.; Socea, B.; Bacalbasa, N.; Surlin, M.V.; Georgescu, I.; et al. Neutrophil/Lymphocyte Ratio as Predictor of Anastomotic Leak after Gastric Cancer Surgery. Diagnostics 2020, 10, 799. [Google Scholar] [CrossRef] [PubMed]
- Abraham, N.S.; Byrne, C.J.; Young, J.M.; Solomon, M.J. Meta–analysis of well–designed nonrandomized comparative studies of surgical procedures is as good as randomized controlled trials. J. Clin. Epidemiol. 2010, 63, 238–245. [Google Scholar] [CrossRef] [PubMed]




| Author, Year, Country | Study Design, Inclusion Criteria | Approach | Pathological Staging | Neo/Adjuvant Chemotherapy | Follow up, Months (Median) | Adjustment |
|---|---|---|---|---|---|---|
| Ha, 2008, Korea | -Retrospective -2004–2006 -Early gastric cancer | Open | ≥pT3: Partial = 0%; Total = 1.5% LN metastasis: Partial = 7.3%; Total = 7.6% | Not mentioned | 20.5 ± 8.6 months (mean + SD) 6.7% loss follow up | None |
| Kim JH, 2009, Korea | -Retrospective -2000/01–2002/12 -Advanced gastric cancer, pT2 | Open | ≥ pT3: Partial = 0%; Total = 0% LN metastasis: Partial = 11.8%; Total = 5% | Not mentioned | Not mentioned | None |
| Kim MC, 2011, Korea | -Retrospective -2005–2006 -Early gastric cancer | Open | ≥ pT3: Partial = Not mentioned; Total = Not mention LN metastasis: Partial = 57.7%; Total = 44.3% | Not mentioned | 38.1 | None |
| Hasegawa, 2013, Japan | - Retrospective with PSM -2001/01–2009/12 -Advanced gastric cancer (adenocarcinoma, pT2–4, N0–3, M0, R0 resection) -Excluded positive peritoneal lavage | Mixed | ≥ pT3: Partial = 65.3%; Total = 69.4% LN metastasis: Partial = 58.2%; Total = 60.2% | Neoadjuvant: Partial = 2/98 Total = 4/98 Adjuvant: Partial = 34/98 Total = 20/98 | Partial: 39.6; Total: 61.2 | Age, gender, p-stage, and extent of lymph node dissection |
| Kim DJ, 2014, Korea | -Retrospective -2004/07–2011/12 -Serosa negative advanced gastric cancer | Laparoscopic | Stage ≥ IIb: Partial = 42.4%; Total = 41.3% LN metastasis: Partial = 48.5%; Total = 50% | Not mentioned | Not mentioned | None |
| Ri, 2020, Japan | - Retrospective with PSM -2006 – 2012, Multi-center -Advanced gastric cancer (cT3–4, any N, pR0) | Open | ≥ pT3: Partial = 52.5%; Total = 51.0% ≥ pN2: Partial = 44.9%; Total = 43.7% | Adjuvant: Partial = 103/263 Total = 98/263 -chemotherapy with S-1 generally | 58.8 | 28 measurable parameters: Age, sex, TNM stage, tumor location, metastasis in lymph node, etc. |
| Sakimaru, 2020, Japan | - Retrospective with PSM -2008/03–2017/08 -Advanced gastric cancer (cT3–4) -Excluded M1 including positive peritoneal lavage | Mixed | ≥ pT3: Partial = 69.9%; Total = 68.5% LN metastasis: Partial = 57.5%; Total = 69.9% | Adjuvant: Partial = 45/73 Total = 49/73 | 59 | variables from preoperative and perioperative findings that could affect outcomes: cTN stage, lymphadenectomy, etc. |
| Murakami, 2021, Japan | - Randomized controlled trial (RCT), Phase II -2011/04–2018/10 -Advanced gastric cancer (cT2–4a, N0–2, M0) | Open | >pT3: Partial = 58.4%; Total = 66.4% LN metastasis: Partial = 57%; Total = 64% | Stage II/III disease (except T1N2–3 or T3N0) patients were recommended to receive oral S1 | Not mention | None |
| Seo, 2021, Korea | - Retrospective with PSM -2003/01–2015/12 -Advanced gastric cancer (pT3 or pT4) | Mixed | pT4a: Partial = 50.7%; Total = 55.6% LN metastasis: Partial = 67.6%; Total = 66.7% | Excluded neoadjuvant patient Adjuvant: Partial = 158/225 Total = 149/225 | 48.2 | Patient clinical demographics (age, sex, cT), perioperative outcomes (surgical approach, resection extent, extent of lymph node dissection), and pathologic outcomes (tumor size, T, N) |
| Study | Selection Q1: Representativeness of Exposed Cohort | Selection Q2: Selection of Non-exposed Cohort | Selection Q3: Ascertainment of Exposure | Selection Q4: Outcome of Interest not Present at Start of Study | Comparability | Outcome Q1: Assessment of Outcome | Outcome Q2: Was Follow-Up Long Enough for Outcome to Occur | Outcome Q3: Adequacy of Follow up of Cohort | Overall (Total 9 points) |
|---|---|---|---|---|---|---|---|---|---|
| Ha, 2008 | V | V | V | V | V | V | 6 | ||
| Kim JH, 2009 | V | V | V | V | V | V | 6 | ||
| Kim MC, 2011 | V | V | V | V | V | V | V | 7 | |
| Hasegawa, 2013 | V | V | V | V | VV | V | V | V | 9 |
| Kim DJ, 2014 | V | V | V | V | V | V | V | 7 | |
| Ri, 2020 | V | V | V | V | VV | V | V | V | 9 |
| Sakimaru, 2020 | V | V | V | V | VV | V | V | V | 9 |
| Seo,2021 | V | V | V | V | VV | V | V | V | 9 |
| Murakami, 2021 | Randomization process: Low risk Deviations from intended interventions: Some concern Missing outcome data: Low risk of bias Measurement of the outcome: Low risk of bias Selection of the reported result: Low risk of bias | Low risk of bias | |||||||
| Outcome | Pooled Effect (95% CI; p Value) | Test for Heterogeneity | Test for Interaction |
|---|---|---|---|
| Overall survival | |||
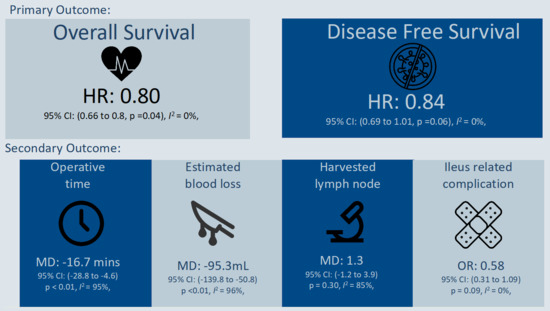
| Overall (6 studies) | HR: 0.80 (0.66 to 0.98, p = 0.04) | I2 = 0%, p = 0.69 | NA |
| Subgroup analysis according to study design | |||
| Analysis without PSM (2 studies) | HR: 0.97 (0.47 to 1.98, p = 0.92) | I2 = 0%, p = 0.34 | p = 0.60 |
| Analysis with PSM (4 studies) | HR: 0.79 (0.64 to 0.98, p = 0.03) | I2 = 0%, p = 0.60 | |
| Disease free survival | |||
| Overall (5 studies) | HR: 0.84 (0.69 to 1.01; p = 0.06) | I2 = 0%, p = 0.62 | NA |
| Subgroup analysis according to study design | |||
| Study with retrospective (1 studies) | HR: 0.54 (0.22 to 1.34, p = 0.18) | NA | p = 0.34 |
| Study with PSM (4 studies) | HR: 0.85 (0.71 to 1.04, p = 0.11 | I2 = 0%, p = 0.64 | |
| Composite outcomes (Clavien-Dindo classification ≥ grade 3) | |||
| Overall (5 studies) | OR: 0.85 (0.60 to 1.21, p = 0.37) | I2 = 50%, p = 0.09 | |
| Complication, adhesion and ileus | |||
| Overall (6 studies) | OR: 0.58 (0.31 to 1.09, p = 0.09) | I2 = 0%, p = 0.50 | |
| Operative time | |||
| Overall (7 studies) | MD: −16.7 mins (−28.8 to −4.6, p < 0.01) | I2 = 95%, p < 0.01 | NA |
| Subgroup analysis according to operation method | |||
| Open method (4 studies) | MD: −2.1 mins (−10.9 to 6.7, p = 0.64) | I2 = 68%, p = 0.02 | p < 0.01 |
| Mixed or MIS method (3 studies) | MD: −32.8 mins (−48.9 to −16.8, p < 0.01) | I2 = 94%, p < 0.01 | |
| Estimated blood loss | |||
| Overall (4 studies) | MD: −95.3 mL (−139.8 to −50.8; p < 0.01) | I2 = 96%, p < 0.01 | |
| Numbers of Lymph nodes harvested | |||
| Overall (6 studies) | MD: 1.3 (−1.2 to 3.9; p = 0.30) | I2 = 85%, p < 0.01 | |
| Length of stay | |||
| Overall (4 studies) | MD: −0.4 (−1.0 to 0.2; p = 0.21) | I2 = 94%, p < 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chai, S.W.; Wang, S.-H.; Wang, C.-Y.; Chen, Y.-C.; Soong, R.-S.; Huang, T.-S. Partial Versus Total Omentectomy in Patients with Gastric Cancer: A Systemic Review and Meta-Analysis. Cancers 2021, 13, 4971. https://doi.org/10.3390/cancers13194971
Chai SW, Wang S-H, Wang C-Y, Chen Y-C, Soong R-S, Huang T-S. Partial Versus Total Omentectomy in Patients with Gastric Cancer: A Systemic Review and Meta-Analysis. Cancers. 2021; 13(19):4971. https://doi.org/10.3390/cancers13194971
Chicago/Turabian StyleChai, Shion Wei, Suo-Hsien Wang, Chih-Yuan Wang, Yi-Chan Chen, Ruey-Shyang Soong, and Ting-Shuo Huang. 2021. "Partial Versus Total Omentectomy in Patients with Gastric Cancer: A Systemic Review and Meta-Analysis" Cancers 13, no. 19: 4971. https://doi.org/10.3390/cancers13194971