Electrochemical Detection of Vibrio cholerae by Amine Functionalized Biocompatible Gadolinium Oxide Nanoparticles
Abstract
:1. Introduction
2. Experimental Techniques
2.1. Materials and Reagents
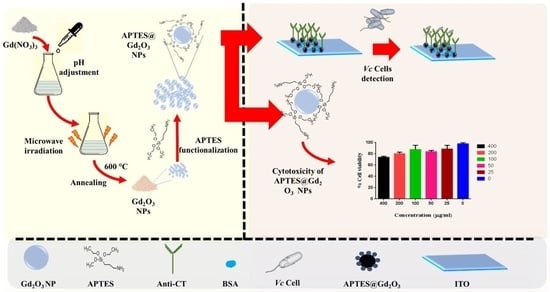
2.2. Synthesis of Gadolinium Oxide (Gd2O3) Nanoparticles
2.3. Functionalization of Gd2O3 with APTES
2.4. Electrophoretic Deposition of Functionalized Gd2O3 on ITO Electrode (APTES@Gd2O3/ITO)
2.5. Immobilization of Anti-CT onto APTES@Gd2O3/ITO Electrode
2.6. In Vitro Cytotoxicity Assay
2.7. Cell Cycle Analysis
2.8. Instrumentation
3. Results and Discussions
3.1. Structural and Morphological Study
3.2. Electrochemical Characterizations
3.3. In Vitro Cytotoxicity Assay
3.4. Cell Cycle Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumar, A.; Purohit, B.; Mahato, K.; Chandra, P. Chapter 11: Advance Engineered Nanomaterials in Point-of-Care Immunosensing for Biomedical Diagnostics. In RSC Detection Science; Royal Society of Chemistry: London, UK, 2019; Volume 2019, pp. 238–266. ISBN 9781788011020. [Google Scholar]
- Purohit, B.; Kumar, A.; Mahato, K.; Chandra, P. Electrodeposition of Metallic Nanostructures for Biosensing Applications in Health Care. J. Sci. Res. 2020, 64, 68–73. [Google Scholar] [CrossRef]
- Kumar, A.; Sharma, S.; Pandey, L.M.; Chandra, P. Nanoengineered Material Based Biosensing Electrodes for Enzymatic Biofuel Cells Applications. Mater. Sci. Energy Technol. 2018, 1, 38–48. [Google Scholar] [CrossRef]
- Roduner, E. Size Matters: Why Nanomaterials Are Different. Chem. Soc. Rev. 2006, 35, 583–592. [Google Scholar] [CrossRef] [PubMed]
- Solanki, P.R.; Kaushik, A.; Agrawal, V.V.; Malhotra, B.D. Nanostructured Metal Oxide-Based Biosensors. NPG Asia Mater. 2011, 3, 17–24. [Google Scholar] [CrossRef]
- Wasim, M.; Mushtaq, M.; Khan, S.U.; Farooq, A.; Naeem, M.A.; Khan, M.R.; Salam, A.; Wei, Q. Development of Bacterial Cellulose Nanocomposites: An Overview of the Synthesis of Bacterial Cellulose Nanocomposites with Metallic and Metallic-Oxide Nanoparticles by Different Methods and Techniques for Biomedical Applications. J. Ind. Text. 2022, 51, 1886S–1915S. [Google Scholar] [CrossRef]
- Ilinskaya, A.N.; Dobrovolskaia, M.A. Immunosuppressive and Anti-Inflammatory Properties of Engineered Nanomaterials. Br. J. Pharmacol. 2014, 171, 3988–4000. [Google Scholar] [CrossRef]
- Carrouel, F.; Viennot, S.; Ottolenghi, L.; Gaillard, C.; Bourgeois, D. Nanoparticles as Anti-Microbial, Anti-Inflammatory, and Remineralizing Agents in Oral Care Cosmetics: A Review of the Current Situation. Nanomaterials 2020, 10, 140. [Google Scholar] [CrossRef]
- Baranwal, A.; Chiranjivi, A.K.; Kumar, A.; Dubey, V.K.; Chandra, P. Design of Commercially Comparable Nanotherapeutic Agent against Human Disease-Causing Parasite, Leishmania. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef]
- Kumar, A.; Purohit, B.; Maurya, P.K.; Pandey, L.M.; Chandra, P. Engineered Nanomaterial Assisted Signal-Amplification Strategies for Enhancing Analytical Performance of Electrochemical Biosensors. Electroanalysis 2019, 31, 1615–1629. [Google Scholar] [CrossRef]
- Kumar, A.; Purohit, B.; Mahato, K.; Roy, S.; Srivastava, A.; Chandra, P. Design and Development of Ultrafast Sinapic Acid Sensor Based on Electrochemically Nanotuned Gold Nanoparticles and Solvothermally Reduced Graphene Oxide. Electroanalysis 2020, 32, 59–69. [Google Scholar] [CrossRef]
- Kumar, A.; Purohit, B.; Mahato, K.; Mandal, R.; Srivastava, A.; Chandra, P. Gold-Iron Bimetallic Nanoparticles Impregnated Reduced Graphene Oxide Based Nanosensor for Label-Free Detection of Biomarker Related to Non-Alcoholic Fatty Liver Disease. Electroanalysis 2019, 31, 2417–2428. [Google Scholar] [CrossRef]
- Ahmad, J.; Majid, K. Improved Thermal Stability Metal Oxide/GO-Based Hybrid Materials for Enhanced Anti-Inflammatory and Antioxidant Activity. Polym. Bull. 2021, 78, 3889–3911. [Google Scholar] [CrossRef]
- Agarwal, H.; Nakara, A.; Shanmugam, V.K. Anti-Inflammatory Mechanism of Various Metal and Metal Oxide Nanoparticles Synthesized Using Plant Extracts: A Review. Biomed. Pharmacother. 2019, 109, 2561–2572. [Google Scholar] [CrossRef] [PubMed]
- Bachheti, R.K.; Godebo, Y.; Bachheti, A.; Yassin, M.O.; Husen, A. Root-Based Fabrication of Metal/Metal-Oxide Nanomaterials and Their Various Applications. Nanomater. Agric. For. Appl. 2020, 2020, 135–166. [Google Scholar]
- Walters, C.; Pool, E.; Somerset, V. Nanotoxicology: A Review. Toxicol. New Asp. This Sci. Conundrum 2016, 10, 5772–6475. [Google Scholar]
- Kumar, A.; Roy, S.; Srivastava, A.; Naikwade, M.M.; Purohit, B.; Mahato, K.; Naidu, V.G.M.; Chandra, P. Nanotherapeutics. In Nanotechnology in Modern Animal Biotechnology: Concepts and Applications; Elsevier: Amsterdam, The Netherlands, 2019; pp. 149–161. ISBN 9780128188231. [Google Scholar]
- Singh, A.V.; Laux, P.; Luch, A.; Sudrik, C.; Wiehr, S.; Wild, A.-M.; Santomauro, G.; Bill, J.; Sitti, M. Review of Emerging Concepts in Nanotoxicology: Opportunities and Challenges for Safer Nanomaterial Design. Toxicol. Mech. Methods 2019, 29, 378–387. [Google Scholar] [CrossRef]
- Bhatia, S.K.; Yetter, A.B. Correlation of Visual in Vitro Cytotoxicity Ratings of Biomaterials with Quantitative in Vitro Cell Viability Measurements. Cell Biol. Toxicol. 2008, 24, 315–319. [Google Scholar] [CrossRef]
- Manuja, A.; Kumar, B.; Kumar, R.; Chhabra, D.; Ghosh, M.; Manuja, M.; Brar, B.; Pal, Y.; Tripathi, B.N.; Prasad, M. Metal/Metal Oxide Nanoparticles: Toxicity Concerns Associated with Their Physical State and Remediation for Biomedical Applications. Toxicol. Rep. 2021, 8, 1970–1978. [Google Scholar] [CrossRef]
- Hazarika, S.; Mohanta, D. Production and Optoelectronic Response of Tb3 Activated Gadolinium Oxide Nanocrystalline Phosphors. Eur. Phys. J. Appl. Phys. 2013, 62, 30401. [Google Scholar] [CrossRef]
- Singh, M.P.; Thakur, C.S.; Shalini, K.; Banerjee, S.; Bhat, N.; Shivashankar, S.A. Structural, Optical, and Electrical Characterization of Gadolinium Oxide Films Deposited by Low-Pressure Metalorganic Chemical Vapor Deposition. J. Appl. Phys. 2015, 96, 5631–5637. [Google Scholar] [CrossRef]
- Rogosnitzky, M.; Branch, S. Gadolinium-Based Contrast Agent Toxicity: A Review of Known and Proposed Mechanisms. BioMetals 2016, 29, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; McDonagh, B.H.; Hak, S.; Peddis, D.; Bandopadhyay, S.; Sandvig, I.; Sandvig, A.; Glomm, W.R. Synthesis of Gadolinium Oxide Nanodisks and Gadolinium Doped Iron Oxide Nanoparticles for MR Contrast Agents. J. Mater. Chem. B 2017, 5, 418–422. [Google Scholar] [CrossRef]
- Gayathri, T.; Sundaram, N.M.; Kumar, R.A. Gadolinium Oxide Nanoparticles for Magnetic Resonance Imaging and Cancer Theranostics. J. Bionanosci. 2016, 9, 409–423. [Google Scholar] [CrossRef]
- Hemmer, E.; Yamano, T.; Kishimoto, H.; Venkatachalam, N.; Hyodo, H.; Soga, K. Cytotoxic Aspects of Gadolinium Oxide Nanostructures for Up-Conversion and NIR Bioimaging. Acta Biomater. 2013, 9, 4734–4743. [Google Scholar] [CrossRef]
- Hazarika, S.; Paul, N.; Mohanta, D. Rapid Hydrothermal Route to Synthesize Cubic-Phase Gadolinium Oxide Nanorods. Bull. Mater. Sci. 2014, 37, 789–796. [Google Scholar] [CrossRef]
- Faucher, L.; Tremblay, M.; Lagueux, J.; Gossuin, Y.; Fortin, M.-A. Rapid Synthesis of PEGylated Ultrasmall Gadolinium Oxide Nanoparticles for Cell Labeling and Tracking with MRI. ACS Appl. Mater. Interfaces 2012, 4, 4506–4515. [Google Scholar] [CrossRef]
- Kumar, A.; Sarkar, T.; Solanki, P.R. Amine Functionalized Gadolinium Oxide Nanoparticles-Based Electrochemical Immunosensor for Cholera. Biosensors 2023, 13, 177. [Google Scholar] [CrossRef]
- Koh, W.C.A.; Chandra, P.; Kim, D.M.; Shim, Y.B. Electropolymerized Self-Assembled Layer on Gold Nanoparticles: Detection of Inducible Nitric Oxide Synthase in Neuronal Cell Culture. Anal. Chem. 2011, 83, 6177–6183. [Google Scholar] [CrossRef]
- Blanco, L.P.; Dirita, V.J. Bacterial-Associated Cholera Toxin and GM 1 Binding Are Required for Transcytosis of Classical Biotype Vibrio Cholerae through an in Vitro M Cell Model System. Cell Microbiol. 2006, 8, 982–998. [Google Scholar] [CrossRef]
- Grela, E.; Kozłowska, J.; Grabowiecka, A. Current Methodology of MTT Assay in Bacteria–A Review. Acta Histochem. 2018, 120, 303–311. [Google Scholar] [CrossRef]
- Cooper, S. Rethinking Synchronization of Mammalian Cells for Cell Cycle Analysis. Cell Mol. Life Sci. 2003, 60, 1099–1106. [Google Scholar] [CrossRef] [PubMed]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, A.; Sarkar, T.; Kumar, R.; Panda, A.K.; Solanki, P.R. Electrochemical Detection of Vibrio cholerae by Amine Functionalized Biocompatible Gadolinium Oxide Nanoparticles. Micromachines 2023, 14, 995. https://doi.org/10.3390/mi14050995
Kumar A, Sarkar T, Kumar R, Panda AK, Solanki PR. Electrochemical Detection of Vibrio cholerae by Amine Functionalized Biocompatible Gadolinium Oxide Nanoparticles. Micromachines. 2023; 14(5):995. https://doi.org/10.3390/mi14050995
Chicago/Turabian StyleKumar, Ashutosh, Tamal Sarkar, Robin Kumar, Amulya K. Panda, and Pratima R. Solanki. 2023. "Electrochemical Detection of Vibrio cholerae by Amine Functionalized Biocompatible Gadolinium Oxide Nanoparticles" Micromachines 14, no. 5: 995. https://doi.org/10.3390/mi14050995