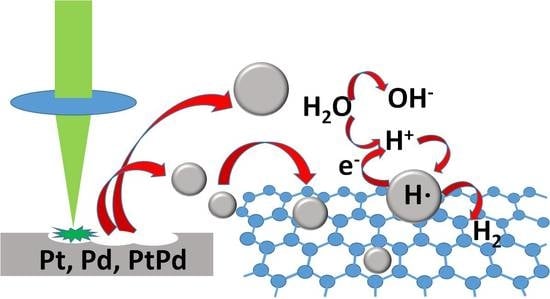
Electro-Sorption of Hydrogen by Platinum, Palladium and Bimetallic Pt-Pd Nanoelectrode Arrays Synthesized by Pulsed Laser Ablation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and NPs-GP Preparation
2.2. Instrumental Characterization
3. Results
3.1. Morphology of NPs-GP Nanoelectrode Arrays
3.2. Structure of PLAL NPs
3.3. Surface and Electronic Structure of NPs
3.4. Electro-Sorption and Galvanostatic Charge-Discharge Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nassar, N.T.; Brainard, J.; Gulley, A.; Manley, R.; Matos, G.; Lederer, G.; Bird, L.R.; Pineault, D.; Alonso, E.; Gambogi, J.; et al. Evaluating the mineral commodity supply risk of the U.S. manufacturing sector. Sci. Adv. 2020, 6, eaay8647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali, S.; Giurco, D.; Arndt, N.; Nickless, E.; Brown, G.; Demetriades, A.; Durrheim, R.; Enriquez, M.; Kinnaird, J.; Littleboy, A.; et al. Mineral supply for sustainable development requires resource governance. Nature 2017, 543, 367–372. [Google Scholar] [CrossRef] [PubMed]
- Boretti, A.; Rosa, L. Reassessing the projections of the World Water Development Report. NPJ Clean Water 2019, 2, 15. [Google Scholar] [CrossRef]
- Chukwudi Tashie-Lewis, B.; Godfrey Nnabuife, S. Hydrogen Production, Distribution, Storage and Power Conversion in a Hydrogen Economy—A Technology Review. J. Adv. Chem. Eng. 2021, 8, 100172. [Google Scholar] [CrossRef]
- Boateng, E.; Chen, A. Recent advances in nanomaterial-based solid-state hydrogen storage. Mater. Today Adv. 2020, 6, 100022. [Google Scholar] [CrossRef]
- Navlani-García, M.; Mori, K.; Kuwahara, Y.; Yamashita, H. Recent strategies targeting efficient hydrogen production from chemical hydrogen storage materials over carbon-supported catalysts. NPG Asia Mater. 2018, 10, 277–292. [Google Scholar] [CrossRef] [Green Version]
- Andersson, J.; Gronkvist, S. Large-scale storage of hydrogen. Int. J. Hydrogen Energy 2019, 44, 11901–11919. [Google Scholar] [CrossRef]
- Li, X.; Zhao, L.; Yu, J.; Liu, X.; Zhang, X.; Liu, H.; Zhou, W. Water Splitting: From Electrode to Green Energy System. Nano-Micro Lett. 2020, 12, 131. [Google Scholar] [CrossRef]
- Mohan, M.; Kumar Sharma, V.; Anil Kumar, E.; Gayathri, V. Hydrogen storage in carbon materials—A review. Energy Storage 2019, 1, e35. [Google Scholar] [CrossRef]
- Wang, H.; Gao, Q.; Hu, J. High Hydrogen Storage Capacity of Porous Carbons Prepared by Using Activated Carbon. J. Am. Chem. Soc. 2009, 131, 7016–7022. [Google Scholar] [CrossRef]
- Schilter, D. Hydrogen Storage: A reformed approach. Nat. Rev. Chem. 2017, 1, 0027. [Google Scholar] [CrossRef]
- Li, J.; Cheng, Y.; Zhang, J.; Fu, J.; Yan, W.; Xu, Q. Confining Pd Nanoparticles and Atomically Dispersed Pd into Defective MoO3 Nanosheet for Enhancing Electro- and Photocatalytic Hydrogen Evolution Performances. ACS Appl. Mater. Interfaces 2019, 31, 27798–27804. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Liu, X.; Zhang, J.; Yan, X.; Liu, Y.; Yuan, A. Enhanced room-temperature hydrogen storage capacity in Pt-loaded graphene oxide/HKUST-1 composites. Int. J. Hydrogen Energy 2014, 39, 2160–2167. [Google Scholar] [CrossRef]
- Faye, O.; Szpunar, J.; Szpunar, B.; Beye, A. Hydrogen adsorption and storage on Palladium—Functionalized graphene with NH-dopant: A first principles calculation. Appl. Surf. Sci. 2017, 392, 362–374. [Google Scholar] [CrossRef]
- Qu, D.; Zhu, X.; Zheng, D.; Zheng, Y.; Liu, D.; Xie, Z.; Tang, H.; Wen, J.; You, X.; Xiao, L.; et al. Improve Electrochemical Hydrogen Insertion on the Carbon Materials Loaded with Pt nano-particles through H spillover. Electrochim. Acta 2015, 174, 400–405. [Google Scholar] [CrossRef]
- Oh, J.; Bathula, H.B.; Park, J.H.; Suh, Y. A sustainable mesoporous palladium-alumina catalyst for efficient hydrogen release from N-heterocyclic liquid organic hydrogen carriers. Commun. Chem. 2019, 2, 68. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.; Wang, X.; Liu, J.; Wang, K.; Jin, S.; Tan, B. Palladium as a Superior Cocatalyst to Platinum for Hydrogen Evolution Using Covalent Triazine Frameworks as a Support. ACS Appl. Mater. Interfaces 2020, 12, 12774–12782. [Google Scholar] [CrossRef]
- Hurley, N.; McGuire, S.C.; Wong, S. Assessing the Catalytic Behavior of Platinum Group Metal-Based Ultrathin Nanowires Using X-ray Absorption Spectroscopy. ACS Appl. Mater. Interfaces 2021, 13, 58253–58260. [Google Scholar] [CrossRef]
- Kobayashi, H.; Yamauchi, M.; Kitagawa, H.; Kubota, Y.; Kato, K.; Takata, M. On the Nature of Strong Hydrogen Atom Trapping Inside Pd Nanoparticles. J. Am. Chem. Soc. 2008, 130, 1828–1829. [Google Scholar] [CrossRef]
- Baig, N.; Kammakakam, I.; Falath, W. Nanomaterials: A review of synthesis methods, properties, recent progress, and challenges. Mater. Adv. 2021, 2, 1821–1871. [Google Scholar] [CrossRef]
- Tsuji, T. Preparation of NPs using laser ablation in liquids: Fundamental aspects and efficient utilization. In Laser Ablation in Liquid: Principles and Applications in the Preparation of Nanomaterials; Yang, G., Ed.; Jenny Stanford Publishing: Singapore, 2012; pp. 2027–2257. ISBN 9789814310956. [Google Scholar]
- Marzun, G.; Streich, C.; Jendrzej, S.; Barcikowski, S.; Wagener, P. Adsorption of colloidal platinum nanoparticles to supports: Charge transfer and effects of electrostatic and steric interactions. Langmuir 2014, 30, 11928–11936. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Oko, D.N.; Garbarino, S.; Imbeault, R.; Chaker, M.; Tavares, A.C.; Guay, D.; Ma, D. Preparation of PtAu alloy colloids by laser ablation in solution and their characterization. J. Phys. Chem. C. 2012, 116, 13413–13420. [Google Scholar] [CrossRef]
- Reichenberger, S.; Marzun, G.; Muhler, M.; Barcikowski, S. Perspective of Surfactant-Free Colloidal Nanoparticles in Heterogeneous Catalysis. ChemCatChem 2019, 11, 4489–4518. [Google Scholar] [CrossRef]
- Zhang, D.; Gökce, B.; Barcikowski, S. Laser Synthesis and Processing of Colloids: Fundamentals and Applications. Chem. Rev. 2017, 117, 3990–4103. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Zhang, L.; Reichenberger, S.; Barcikowski, S. Design and perspective of amorphous metal nanoparticles from laser synthesis and processing. Phys. Chem. Chem. Phys. 2021, 23, 11121–11154. [Google Scholar] [CrossRef]
- Semaltianos, N.G. Nanoparticles by laser ablation of bulk target materials in liquids. In Handbook of Nanoparticles; Aliofkhazraei, M., Ed.; Springer: Cham, Switzerland, 2016; pp. 67–92. [Google Scholar] [CrossRef]
- Hodges, A.; Hoang, A.; Tsekouras, G.; Wagner, K.; Lee, C.-Y.; Swiegers, G.F.; Wallace, G. A high-performance capillary-fed electrolysis cell promises more cost-competitive renewable hydrogen. Nat. Commun. 2022, 13, 1304. [Google Scholar] [CrossRef]
- Forsythe, R.C.; Cox, C.P.; Wilsey, M.K.; Müller, A.M. Pulsed Laser in Liquids Made Nanomaterials for Catalysis. Chem. Rev. 2021, 121, 7568–7637. [Google Scholar] [CrossRef]
- Censabella, M.; Torrisi, V.; Boninelli, S.; Bongiorno, C.; Grimaldi, M.G.; Ruffino, F. Laser ablation synthesis of mono- and bimetallic Pt and Pd nanoparticles and fabrication of Pt-Pd/Graphene nanocomposites. Appl. Surf. Sci. 2019, 475, 494–503. [Google Scholar] [CrossRef]
- Ozmaiana, M.; Naghdabadi, R. Modeling and simulation of the water gradient within a Nafion membrane. Phys. Chem. Chem. Phys. 2014, 16, 3173. [Google Scholar] [CrossRef]
- Briggs, D.; Grant, J.T. Surface Analysis by Auger and X-ray Photoelectron Spectroscopy; IM Publications: Chichester, UK; Surface Spectra Ltd.: Manchester, UK, 2003. [Google Scholar]
- Gulino, A. Structural and electronic characterization of self-assembled molecular nanoarchitectures by X-ray photoelectron spectroscopy. Anal. Bioanal. Chem. 2013, 405, 1479–1495. [Google Scholar] [CrossRef]
- Cristaldi, D.A.; Millesi, S.; Crupi, I.; Impellizzeri, G.; Priolo, F.; Jacobs, R.M.J.; Egdell, R.G.; Gulino, A. Structural, Electronic, and Electrical Properties of an Undoped n-Type CdO Thin Film with High Electron Concentration. J. Phys. Chem. C 2014, 118, 15019–15026. [Google Scholar] [CrossRef]
- Thompson, M. Xrump. Available online: www.genplot.com (accessed on 22 May 2021).
- Cuynet, S.; Caillard, A.; Lecas, T.; Bigarré, J.; Buvat, P.; Brault, P. Deposition of Pt inside fuel cell electrodes using high power impulse magnetron sputtering. J. Phys. D Appl. Phys. 2014, 47, 272001. [Google Scholar] [CrossRef]
- Howe, J.; Rawn, C.; Jones, L.; Ow, H. Improved crystallographic data for graphite. Powder Diffr. 2003, 18, 150–154. [Google Scholar] [CrossRef]
- Rodríguez-Proenza, C.A.; Palomares-Báez, J.P.; Chávez-Rojo, M.A.; García-Ruiz, A.F.; Azanza-Ricardo, C.L.; Santoveña-Uribe, A.; Luna-Bárcenas, G.; Rodríguez-López, J.L.; Esparza, R. Atomic Surface Segregation and Structural Characterization of PdPt Bimetallic Nanoparticles. Materials 2018, 11, 1882. [Google Scholar] [CrossRef] [Green Version]
- Higareda, A.; Rosas, G.; Pérez, R.; Esparza, R. Characterization and Electrocatalytic Features of PtPd and PdPt Bimetallic Nanoparticles for Methanol Electro-oxidation. ChemNanoMat 2021, 7, 958. [Google Scholar] [CrossRef]
- Spitaleri, L.; Gangemi, C.; Purrello, R.; Nicotra, G.; Trusso Sfrazzetto, G.; Casella, G.; Casarin, M.; Gulino, A. Covalently Conjugated Gold–Porphyrin Nanostructures. Nanomaterials 2020, 10, 1644. [Google Scholar] [CrossRef]
- Zimbone, M.; Cacciato, G.; Spitaleri, L.; Egdell, R.G.; Grimaldi, M.G. A Gulino, Sb-doped titanium oxide: A rationale for its photocatalytic activity for environmental remediation. ACS Omega 2018, 3, 11270–11277. [Google Scholar] [CrossRef] [Green Version]
- Guo, K.; Rowland, L.J.; Isherwood, L.H.; Glodan, G.; Baidak, A. Photon-induced synthesis of ultrafine metal nanoparticles on graphene as electrocatalysts: Impact of functionalization and doping. J. Mater. Chem. A 2020, 8, 714. [Google Scholar] [CrossRef]
- Fiorenza, R.; Spitaleri, L.; Gulino, A.; Scirè, S. Ru–Pd Bimetallic Catalysts Supported on CeO2-MnOX Oxides as Efficient Systems for H2 Purification through CO Preferential Oxidation. Catalysts 2018, 8, 203. [Google Scholar] [CrossRef] [Green Version]
- Zheng, J.-N.; Lv, J.-J.; Li, S.-S.; Xue, M.-W.; Wang, A.-J.; Feng, J.-J. One-pot synthesis of reduced graphene oxide supported hollow Ag@Pt core–shell nanospheres with enhanced electrocatalytic activity for ethylene glycol oxidation. J. Mater. Chem. A 2014, 2, 3445. [Google Scholar] [CrossRef]
- Yen, M.-Y.; Teng, C.-C.; Hsiao, M.-C.; Liu, P.-I.; Chuang, W.-P.; Ma, C.M.; Hsieh, C.-K.; Tsai, M.-C.; Tsai, C.-H. Platinum nanoparticles/graphene composite catalyst as a novel composite counter electrode for high performance dye-sensitized solar cells. J. Mater. Chem. 2011, 21, 12880–12888. [Google Scholar] [CrossRef]
- Gao, G.; Liu, D.; Tang, S.; Huang, C.; He, M.; Guo, Y.; Sun, X.; Gao, B. Heat-Initiated Chemical Functionalization of Graphene. Sci. Rep. 2016, 6, 20034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johra, F.T.; Lee, J.-W.; Jung, W.-G. Facile and safe graphene preparation on solution based platform. J. Ind. Eng. Chem. 2014, 20, 2883–2887. [Google Scholar] [CrossRef]
- Giofrè, S.V.; Tiecco, M.; Celesti, C.; Patanè, S.; Triolo, C.; Gulino, A.; Spitaleri, L.; Scalese, S.; Scuderi, M.; Iannazzo, D. Eco-Friendly 1,3-Dipolar Cycloaddition Reactions on Graphene Quantum Dots in Natural Deep Eutectic Solvent. Nanomaterials 2020, 10, 2549. [Google Scholar] [CrossRef]
- Militello, M.C.; Simko, S.J. Elemental Palladium by XPS. Surf. Sci. Spectra 1994, 3, 387. [Google Scholar] [CrossRef]
- Yuan, L.; Liu, S.; Xu, S.; Yang, X.; Bian, J.; Lv, C.; Yu, Z.; He, T.; Huang, Z.; Boukhvalov, D.W.; et al. Modulation of Volmer step for efficient alkaline water splitting implemented by titanium oxide promoting surface reconstruction of cobalt carbonate hydroxide. Nano Energy 2021, 82, 105732. [Google Scholar] [CrossRef]
- Kronberg, R.; Lappalainen, H.; Laasonen, K. Revisiting the Volmer–Heyrovsky´ mechanism of hydrogen evolution on a nitrogen doped carbon nanotube: Constrained molecular dynamics versus the nudged elastic band method. Phys. Chem. Chem. Phys. 2020, 22, 10536. [Google Scholar] [CrossRef] [Green Version]
- Dickinson, E.; Wain, A.J. The Butler-Volmer equation in electrochemical theory: Origins, value, and practical application. J. Electroanal. Chem. 2020, 872, 114145. [Google Scholar] [CrossRef]
- Farfour, N.; Chbihi, M.; Takky, D.; Eddahaoui, K.; Benmokhtar, S. Catalytic oxidation of methanol on Pt/X (X = CaTP, NaTP) electrodes in sulfuric acid solution. Mediterr. J. Chem. 2013, 2, 595–606. [Google Scholar] [CrossRef]
- Zhan, D.; Velmurugan, J.; Mirkin, M.V. Adsorption/Desorption of Hydrogen on Pt Nanoelectrodes: Evidence of Surface Diffusion and Spillover. J. Am. Chem. Soc. 2009, 131, 14756–14760. [Google Scholar] [CrossRef]
- Welch, C.M.; Compton, R.G. The use of nanoparticles in electroanalysis: A review. Anal. Bioanal. Chem. 2006, 384, 601–619. [Google Scholar] [CrossRef] [PubMed]
- Oberoi, A.S.; Nijhawan, P.; Singh, P. A Novel Electrochemical Hydrogen Storage-Based Proton Battery for Renewable Energy Storage. Energies 2019, 12, 82. [Google Scholar] [CrossRef] [Green Version]
- Mosavati, N.; Young, K.-H.; Meng, T.; Simon Ng, K.Y. Electrochemical Open-Circuit Voltage and Pressure-Concentration-Temperature Isotherm Comparison for Metal Hydride Alloys. Batteries 2016, 2, 6. [Google Scholar] [CrossRef] [Green Version]
- Boateng, E.; Dondapati, J.S.; Thiruppathi, A.R.; Chen, A. Significant enhancement of the electrochemical hydrogen uptake of reduced graphene oxide via boron-doping and decoration with Pd nanoparticles. Int. J. Hydrogen Energy 2020, 45, 28951–28963. [Google Scholar] [CrossRef]
- Dubouis, N.; Grimaud, A. The hydrogen evolution reaction: From material to interfacial descriptors. Chem. Sci. 2019, 10, 9165–9181. [Google Scholar] [CrossRef] [Green Version]
- Wei, L.; Mao, Y. Enhanced hydrogen storage performance of reduced graphene oxide hybrids with nickel or its metallic mixtures based on spillover mechanism. Int. J. Hydrogen Energy 2016, 41, 11692–11699. [Google Scholar] [CrossRef] [Green Version]
- Mehrabi, M.; Parvin, P.; Reyhani, A.; Mortazavi, S.Z. Hydrogen storage in multi-walled carbon nanotubes decorated with palladium nanoparticles using laser ablation/chemical reduction methods. Mater. Res. Express 2017, 4, 095030. [Google Scholar] [CrossRef] [Green Version]








| Sample | C 1s | O 1s | Pt 4f7/2,5/2 | Pd 3d5/2,3/2 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| C sp2 | C-OH | C=O | O-C=O | C-OxH | H2O | Pt0 | Pt(II) | Pt (IV) | Pd0 | Pd(II) | |
| Pd-GP | 284.6 | 285.8 | 287.1 | 288.4 | 531.5 | 535.5 | - | - | - | 335.5/340.8 | 337.5/342.8 |
| Pt-GP | 284.6 | 286.0 | 287.1 | 288.3 | 531.1 | 533.1 | 71.3/74.6 | 72.6/76.0 | 75.2/78.5 | - | - |
| Pt80Pd20GP | 284.6 | 285.7 | 286.9 | 288.5 | 531.8 | 534.0 | 71.3/74.6 | 72.5/75.9 | 75.0/78.3 | 335.5/340.8 | 336.8/342.1 |
| Sample | C 1s (tot.) | O 1s (tot.) | Pt 4f7/2,5/2 Pt0+Pt(II)+Pt(IV) | Pd 3d3/2,1/2 Pd0+Pd(II) |
|---|---|---|---|---|
| Pd | 75.5 | 22.9 | - | 1.6 |
| Pt | 49.2 | 20.9 | 29.9 | - |
| Pt80Pd20 | 80.3 | 16.4 | 2.0 | 1.3 |
| System | Suspension Medium of NPs | Charge (−0.3 V) | Discharge (+0.25 V) | Faradaic Efficiency % |
|---|---|---|---|---|
| GP alone | - | 4.65 | 2.77 | 59.6 |
| Pt-GP | water | 4.64 | 2.98 | 64.2 |
| Pd-GP | water | 5.60 | 3.70 | 66.1 |
| Pt80Pd20-GP | water | 5.16 | 4.02 | 77.9 |
| Pt-GP | water-0.25 wt.% nafion | 5.60 | 4.85 | 86.6 |
| Pd-GP | water-0.25 wt.% nafion | 5.77 | 2.76 | 47.8 |
| Pt80Pd20-GP | water-0.25 wt.% nafion | 6.21 | 4.50 | 72.4 |
| System | Electrode | Metal NPs Production Method | Electrolyte (ElectroChemical Method) or Gaseous Phase Reaction of H2 Adsorption | Hydrogen Storage/Evolution (Wt.%) | Faradaic Efficiency (%) | Reference |
|---|---|---|---|---|---|---|
| Pd NPs/nafion | GCE | Wet/NaBH4 | H2SO4 | 0.003 | 83.1 | [52] |
| Pd-rGO/nafion | GCE | Wet/NaBH4 | H2SO4 | 0.14 | 85 | [52] |
| Pd/B-rGO/nafion | GCE | Wet/NaBH4 | H2SO4 | 0.35 | 95 | [52] |
| Pt- (GO)/HKUST-1 | - | Wet/Ethylene glycol | Gaseous phase reaction | 1.6 | - | [12] |
| Pt Covalent triazine framework(CTF-N) | Fluorine doped tin oxide (FTO) | Wet/NaBH4 | Trietanolamine (1) | 0.2 | - | [16] |
| Pd Covalent triazine framework(CTF-N) | Fluorine doped tin oxide (FTO) | Wet/NaBH4 | Trietanolamine (1) | 1.05 | - | [16] |
| Ni/rGO | - | Reduction in H2 (2) | Gaseous phase reaction | 0.007 | - | [60] |
| Ni/Pd/rGO, | - | Reduction in H2 (2) | Gaseous phase reaction | 0.13 | - | [60] |
| Ni/Ag/Pd/rGO | - | Reduction in H2 (2) | Gaseous phase reaction | 0.055 | - | [60] |
| Pd/graphene | - | Reduction in H2 (2) | Gaseous phase reaction | 8.67 (3) | - | [4] |
| Pd/MWCNT | - | PLAL | Gaseous phase reaction | 1.2 | - | [61] |
| Pt-GP | GP | PLAL | KOH | 1 | 64.2 | This work |
| Pd-GP | GP | PLAL | KOH | 6 | 66.1 | This work |
| Pt80Pd20-GP | GP | PLAL | KOH | 6 | 77.9 | This work |
| Pt-GP/nafion | GP | PLAL | KOH | 1 | 86.6 | This work |
| Pd-GP/nafion | GP | PLAL | KOH | 6 | 47.8 | This work |
| Pt80Pd20-GP/nafion | GP | PLAL | KOH | 6 | 72.4 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scandurra, A.; Censabella, M.; Gulino, A.; Grimaldi, M.G.; Ruffino, F. Electro-Sorption of Hydrogen by Platinum, Palladium and Bimetallic Pt-Pd Nanoelectrode Arrays Synthesized by Pulsed Laser Ablation. Micromachines 2022, 13, 963. https://doi.org/10.3390/mi13060963
Scandurra A, Censabella M, Gulino A, Grimaldi MG, Ruffino F. Electro-Sorption of Hydrogen by Platinum, Palladium and Bimetallic Pt-Pd Nanoelectrode Arrays Synthesized by Pulsed Laser Ablation. Micromachines. 2022; 13(6):963. https://doi.org/10.3390/mi13060963
Chicago/Turabian StyleScandurra, Antonino, Maria Censabella, Antonino Gulino, Maria Grazia Grimaldi, and Francesco Ruffino. 2022. "Electro-Sorption of Hydrogen by Platinum, Palladium and Bimetallic Pt-Pd Nanoelectrode Arrays Synthesized by Pulsed Laser Ablation" Micromachines 13, no. 6: 963. https://doi.org/10.3390/mi13060963